2'-HydroxygenisteinCAS# 1156-78-1 |
Quality Control & MSDS
Number of papers citing our products

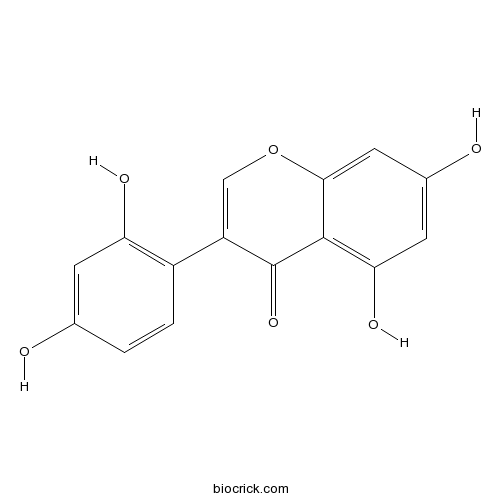
Chemical structure

3D structure
| Cas No. | 1156-78-1 | SDF | Download SDF |
| PubChem ID | 5282074 | Appearance | Yellow powder |
| Formula | C15H10O6 | M.Wt | 286.2 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-(2,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one | ||
| SMILES | C1=CC(=C(C=C1O)O)C2=COC3=CC(=CC(=C3C2=O)O)O | ||
| Standard InChIKey | GSSOWCUOWLMMRJ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 2'-Hydroxygenistein has antifungal activity, dimerization of it causes a remarkable increase of antifungal activity. 2. 2'-Hydroxygenistein exhibits greater antiproliferative effects in MCF-7 human breast cancer cells than does genistein, suggest that 2'-hydroxylation of genistein can enhance its antioxidant activity and cell cytotoxicity in MCF-7 human breast cancer cells. 3. 2'-Hydroxygenistein shows significant concentration-dependent inhibitory effects on the release of beta-glucuronidase and lysozyme from rat neutrophils in response to formyl-Met-Leu-Phe/cytochalasin B. |
| Targets | Antifection |

2'-Hydroxygenistein Dilution Calculator

2'-Hydroxygenistein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4941 mL | 17.4703 mL | 34.9406 mL | 69.8812 mL | 87.3515 mL |
| 5 mM | 0.6988 mL | 3.4941 mL | 6.9881 mL | 13.9762 mL | 17.4703 mL |
| 10 mM | 0.3494 mL | 1.747 mL | 3.4941 mL | 6.9881 mL | 8.7352 mL |
| 50 mM | 0.0699 mL | 0.3494 mL | 0.6988 mL | 1.3976 mL | 1.747 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3494 mL | 0.6988 mL | 0.8735 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Marbofloxacin hydrochloride
Catalog No.:BCC4250
CAS No.:115551-26-3
- Marbofloxacin
Catalog No.:BCC2510
CAS No.:115550-35-1
- BI 224436
Catalog No.:BCC5531
CAS No.:1155419-89-8
- Fmoc-Cys(Trt)-Opfp
Catalog No.:BCC3480
CAS No.:115520-21-3
- (2-Amino-1-hydroxyethyl)phosphonic acid
Catalog No.:BCN1613
CAS No.:115511-00-7
- H-Lys(Z)-OH
Catalog No.:BCC2986
CAS No.:1155-64-2
- Z-Glu-OH
Catalog No.:BCC2781
CAS No.:1155-62-0
- 5,6,7,7a-Tetrahydrothieno[3,2-c]pyridine-2(4H)-one hydrochloride
Catalog No.:BCC8721
CAS No.:115473-15-9
- Glaucin B
Catalog No.:BCN6035
CAS No.:115458-73-6
- Sootepin D
Catalog No.:BCN6034
CAS No.:1154518-97-4
- Risedronate Sodium
Catalog No.:BCC2501
CAS No.:115436-72-1
- Niloticin
Catalog No.:BCN6033
CAS No.:115404-57-4
- 4-Androstenediol
Catalog No.:BCC8692
CAS No.:1156-92-9
- Cinnamyl coumarate
Catalog No.:BCN7739
CAS No.:115610-30-5
- Cinnamyl isoferulate
Catalog No.:BCN7718
CAS No.:115610-31-6
- Cinnamyl caffeate
Catalog No.:BCN7721
CAS No.:115610-32-7
- H-DL-Pro-NH2
Catalog No.:BCC3027
CAS No.:115630-49-4
- B2
Catalog No.:BCC7505
CAS No.:115687-05-3
- Daidzein dimethyl ether
Catalog No.:BCN6761
CAS No.:1157-39-7
- Adonifoline
Catalog No.:BCN2056
CAS No.:115712-88-4
- 6'-O-beta-D-Glucosylgentiopicroside
Catalog No.:BCN2814
CAS No.:115713-06-9
- 1-O-galloyl-6-O-cinnamoylglucose
Catalog No.:BCN8264
CAS No.:115746-69-5
- Galanolactone
Catalog No.:BCN6037
CAS No.:115753-79-2
- 15,16-Dihydro-15-methoxy-16-oxohardwickiic acid
Catalog No.:BCN1612
CAS No.:115783-35-2
Anti-inflammatory flavonoids and pterocarpanoid from Crotalaria pallida and C. assamica.[Pubmed:15013012]
Bioorg Med Chem Lett. 2004 Feb 23;14(4):1011-4.
One new isoflavone, 5,7,4'-trihydroxy-2'-methoxyisoflavone (3) and seven, and four known compounds were isolated from the barks of Crotalaria pallida and the seeds of C. assamica, respectively. The known compounds, apigenin (1) and 2'-hydroxygenistein (2), isolated from C. pallida, showed significant concentration-dependent inhibitory effects on the release of beta-glucuronidase and lysozyme from rat neutrophils in response to formyl-Met-Leu-Phe/cytochalasin B (fMLP/CB) with IC(50) values of 2.8+/-0.1 and 17.7+/-1.9, and 5.9+/-1.4 and 9.7+/-3.5 microM, respectively. The known compounds, daidzein (4) and 2'-hydroxydaidzein (6), isolated from C. pallida, inhibited of the release of lysozyme and beta-glucuronidase from rat neutrophils in response to fMLP/CB with IC(50) values of 26.3+/-5.5 and 13.7+/-2.6 microM, respectively. Compounds 1 and 4 also showed significant concentration-dependent inhibitory effects on superoxide anion generation in rat neutrophils stimulated with fMLP/CB with IC(50) values of 3.4+/-0.3 and 25.1+/-5.0 microM, respectively. Compounds 1 and 5, previously isolated from C. pallida, showed the inhibition of NO production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages and LPS/interferon-gamma (IFN-gamma)-stimulated N9 microglial cells with IC(50) values of 10.7+/-0.1 and 13.9+/-1.1 microM, respectively. Flavonoids, suppressed chemical mediators in inflammatory cells, may have value in treatment and prevention of central and peripheral inflammatory diseases associated with excess production of chemical mediators.
A new class of biflavonoids: 2'-hydroxygenistein dimers from the roots of white lupin.[Pubmed:10817204]
Z Naturforsch C. 2000 Mar-Apr;55(3-4):165-74.
Two novel isoflavonoid dimers presumably originating from 2'-hydroxygenistein, 5,7,4'-trihydroxycoumaranochroman-4-one-(3-->5"')-5",7",2"'4"'- tetrahydroxyisoflavone (1, lupinalbisone A) and 5,7,4'-trihydroxycoumaranochroman-4-one-(3-6")-5",7",2"',4"'-te trahydroxyisoflavone (2, lupinalbisone B) were isolated from the roots of Lupinus albus L., and their structures involving relative stereochemistry were elucidated by spectroscopic methods. Using horse radish peroxidase and 2'-hydroxygenistein (3) as the substrate revealed the formation of these dimers together with 5,7,4'-trihydroxycoumaronochromone (4, lupinalbin A). Dimerization of 3 caused a remarkable increase of antifungal activity.
2'-hydroxylation of genistein enhanced antioxidant and antiproliferative activities in mcf-7 human breast cancer cells.[Pubmed:19996686]
J Microbiol Biotechnol. 2009 Nov;19(11):1348-54.
Bioconversion of the isoflavonoid genistein to 2'- hydroxygenistein (2'-HG) was performed using isoflavone 2'-hydroxylase (CYP81E1) heterologously expressed in yeast. A monohydroxylated product was analyzed by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) and NMR spectrometry and was identified as 2'-HG. An initial bioconversion rate of 6% was increased up to 14% under optimized conditions. After recovery, the biological activity of 2'-HG was evaluated. Bioconverted 2'-HG showed higher antioxidant activity against 1,1- diphenyl-2-picryl hydrazine (DPPH) and 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radicals than did genistein. Furthermore, 2'-HG exhibited greater antiproliferative effects in MCF-7 human breast cancer cells than did genistein. These results suggest that 2'- hydroxylation of genistein enhanced its antioxidant activity and cell cytotoxicity in MCF-7 human breast cancer cells.
Antiplasmodial constituents of Cajanus cajan.[Pubmed:15022164]
Phytother Res. 2004 Feb;18(2):128-30.
Bioactivity-guided fractionation of extracts of roots and leaves of Cajanus cajan afforded 8 compounds: betulinic acid, biochanin A, cajanol, genistein and 2'-hydroxygenistein, longistylin A and C, and pinostrobin. The two stilbenes, longistylin A and C, and betulinic acid showed a moderately high in vitro activity against the chloroquine-sensitive Plasmodium falciparum strain 3D7.


