AprotininInhibitor of bovine pancreatic trypsin CAS# 9087-70-1 |
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 9087-70-1 | SDF | Download SDF |
| PubChem ID | 53487898 | Appearance | Powder |
| Formula | C284H432N84O79S7 | M.Wt | 6511.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Antilysin | ||
| Solubility | H2O : 100 mg/mL (15.36 mM; Need ultrasonic) | ||
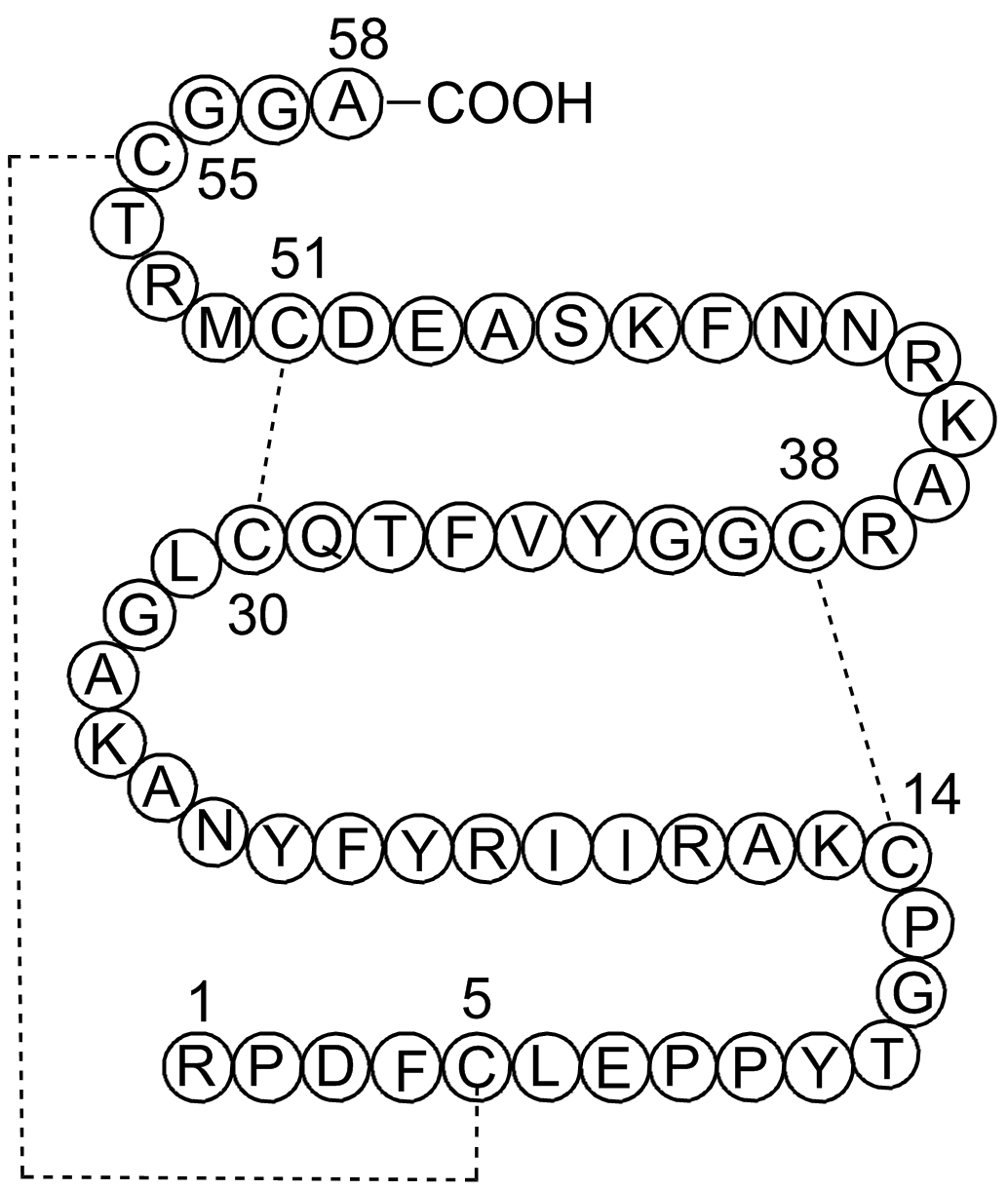
| Sequence | RPDFCLEPPYTGPCKARIIRYFYNAKAGLC (Modifications: Disulfide bridge: 5-55, 14-38, 30-51) | ||
| SMILES | CCC(C)C1C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC2CSSCC3C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)N4CCCC4C(=O)N5CCCC5C(=O)NC(C(=O)NC(C(=O)NCC(=O)N6CCCC6C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N3)CC(=O)O)CCC(=O)O)C)CO)CCCCN)CC7=CC=CC=C7)CC(=O)N)CC(=O)N)CCCNC(=N)N)CCCCN)C)CCCNC(=N)N)NC(=O)CNC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC2=O)CCC(=O)N)C(C)O)CC8=CC=CC=C8)C(C)C)CC9=CC=C(C=C9)O)C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCNC(=N)N)C)CCCCN)C(C)O)CC1=CC=C(C=C1)O)CCC(=O)O)CC(C)C)NC(=O)C(CC1=CC=CC=C1)NC(=O)C(CC(=O)O)NC(=O)C1CCCN1C(=O)C(CCCNC(=N)N)N)C(=O)NCC(=O)NCC(=O)NC(C)C(=O)O)C(C)O)CCCNC(=N)N)CCSC)CC(C)C)C)CCCCN)C)CC(=O)N)CC1=CC=C(C=C1)O)CC1=CC=CC=C1)CC1=CC=C(C=C1)O)CCCNC(=N)N)C(C)CC | ||
| Standard InChIKey | ZPNFWUPYTFPOJU-YSFZTAPISA-N | ||
| Standard InChI | InChI=1S/C284H432N84O79S7/c1-21-144(9)222-271(439)337-174(68-46-105-309-282(300)301)239(407)340-187(120-160-77-85-164(374)86-78-160)251(419)341-185(116-156-55-29-24-30-56-156)250(418)342-188(121-161-79-87-165(375)88-80-161)252(420)346-191(123-208(291)378)246(414)322-149(14)230(398)326-168(62-35-39-98-285)234(402)319-146(11)227(395)314-132-215(385)324-181(113-141(3)4)247(415)354-199-137-452-453-138-200-263(431)336-179(97-112-448-20)242(410)331-176(70-48-107-311-284(304)305)244(412)363-226(154(19)372)274(442)358-197(233(401)316-129-212(382)312-130-213(383)318-151(16)278(446)447)135-449-451-139-201(355-253(421)186(117-157-57-31-25-32-58-157)344-256(424)195(127-220(393)394)350-267(435)204-72-50-109-366(204)275(443)167(289)61-43-102-306-279(294)295)265(433)339-182(114-142(5)6)248(416)338-180(93-96-218(389)390)276(444)368-111-52-74-206(368)277(445)367-110-51-73-205(367)268(436)349-189(122-162-81-89-166(376)90-82-162)259(427)362-224(152(17)370)269(437)317-133-216(386)365-108-49-71-203(365)266(434)357-202(264(432)333-169(63-36-40-99-286)235(403)320-148(13)229(397)328-175(69-47-106-310-283(302)303)243(411)360-223(145(10)22-2)272(440)361-222)140-454-450-136-198(325-214(384)131-313-211(381)128-315-232(400)183(119-159-75-83-163(373)84-76-159)351-270(438)221(143(7)8)359-258(426)190(118-158-59-33-26-34-60-158)352-273(441)225(153(18)371)364-245(413)177(335-262(199)430)91-94-207(290)377)261(429)334-172(66-44-103-307-280(296)297)236(404)321-147(12)228(396)327-170(64-37-41-100-287)237(405)330-173(67-45-104-308-281(298)299)238(406)345-192(124-209(292)379)255(423)347-193(125-210(293)380)254(422)343-184(115-155-53-27-23-28-54-155)249(417)332-171(65-38-42-101-288)240(408)353-196(134-369)260(428)323-150(15)231(399)329-178(92-95-217(387)388)241(409)348-194(126-219(391)392)257(425)356-200/h23-34,53-60,75-90,141-154,167-206,221-226,369-376H,21-22,35-52,61-74,91-140,285-289H2,1-20H3,(H2,290,377)(H2,291,378)(H2,292,379)(H2,293,380)(H,312,382)(H,313,381)(H,314,395)(H,315,400)(H,316,401)(H,317,437)(H,318,383)(H,319,402)(H,320,403)(H,321,404)(H,322,414)(H,323,428)(H,324,385)(H,325,384)(H,326,398)(H,327,396)(H,328,397)(H,329,399)(H,330,405)(H,331,410)(H,332,417)(H,333,432)(H,334,429)(H,335,430)(H,336,431)(H,337,439)(H,338,416)(H,339,433)(H,340,407)(H,341,419)(H,342,418)(H,343,422)(H,344,424)(H,345,406)(H,346,420)(H,347,423)(H,348,409)(H,349,436)(H,350,435)(H,351,438)(H,352,441)(H,353,408)(H,354,415)(H,355,421)(H,356,425)(H,357,434)(H,358,442)(H,359,426)(H,360,411)(H,361,440)(H,362,427)(H,363,412)(H,364,413)(H,387,388)(H,389,390)(H,391,392)(H,393,394)(H,446,447)(H4,294,295,306)(H4,296,297,307)(H4,298,299,308)(H4,300,301,309)(H4,302,303,310)(H4,304,305,311)/t144-,145+,146-,147-,148-,149-,150+,151-,152+,153-,154-,167-,168+,169+,170+,171+,172+,173+,174-,175+,176-,177+,178-,179+,180+,181-,182-,183+,184-,185+,186+,187-,188-,189-,190+,191+,192-,193+,194+,195-,196-,197+,198-,199-,200-,201-,202-,203+,204+,205+,206-,221-,222-,223+,224-,225+,226+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive serine protease inhibitor. Reversibly binds to and blocks the enzymatic active site. Inhibits a range of serine proteases including trypsin, chymotrypsin, kallikrein and plasmin. |

Aprotinin Dilution Calculator

Aprotinin Molarity Calculator
Abstract
Aprotinin and EACA were evaluated for blood-sparing efficacy and other major clinical outcome criteria in infants undergoing cardiac surgery.
Abstract
The associations of aprotinin and red blood cells transfusion, renal injury and mortality in pediatric with cardiac surgery were assessed.
Abstract
Aprotinin reduced PCT level as well as levels of a few inflammatory cytokines, including TNFalpha, IL-1beta, IL-6 and IL-8, in post-PEA patients, in which PCT was significantly correlated with IL-6.
Abstract
Aprotinin may cause adverse effects, including increased risk of mortality and morbidity, during cardiac surgery.
Abstract
A new meta-analysis was conducted to assess safety and efficacy of aprotinin in cardiac surgery.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aprotinin, a naturally occurring serine protease inhibitor, saves lives and decreases the risk of stroke and repeat surgery for massive bleeding1, 2, 3.
The use of aprotinin did not significantly increase the risk of renal failure or the need for postoperative renal replacement despite an increase in the proportion of patients who had a doubling of serum creatinine levels. The adjudication of death did not identify renal failure as contributing to or causing death associated with aprotinin use. A Meta analysis by Brown and colleagues showed a nonsignificant relative risk of renal failure with high-dose aprotinin4.
Although aprotinin is potentially more effective than other active agents in controlling hemostasis, we noted only a possible trend suggesting that it decreased massive bleeding. Only repeat surgeries and important blood losses through chest tubes, one of the main indications for surgery, were potentially improved by the use of aprotinin. Aprotinin did not appear to prevent massive bleeding or save the life of patients who had massive bleeding.
The adverse effects on mortality associated with aprotinin may also have been present among healthier patients, those under the age of 65 years, and those without coexisting illnesses at the time of surgery.
Despite the possibility of a modest reduction in the risk of massive bleeding, the strong and consistent negative mortality trend associated with aprotinin as compared with lysine analogues precludes its use in patients undergoing high-risk cardiac surgery5.
Reference:
1. Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2007;4:CD001886.
2. Levi M, Cromheecke ME, de Jonge E, et al. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet 1999;354:1940-7.
3. Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg 2004;128:442-8.
4. Brown JR, Birkmeyer NJ, O’Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation 2007;115:2801-13.
5. Dean A. Fergusson, Paul C. Hébert et al, A Comparison of Aprotinin and Lysine Analogues in High-Risk Cardiac Surgery, N Engl J Med 2008; 358:2319-2331
- Ptelatoside B
Catalog No.:BCN4451
CAS No.:90852-99-6
- ent-Labda-8(17),13E-diene-3beta,15,18-triol
Catalog No.:BCN7662
CAS No.:90851-50-6
- Goshonoside F5
Catalog No.:BCN6442
CAS No.:90851-28-8
- Goshonoside F1
Catalog No.:BCN6444
CAS No.:90851-24-4
- Cyclo(Ile-Ala)
Catalog No.:BCN2429
CAS No.:90821-99-1
- PF-04929113 (SNX-5422)
Catalog No.:BCC2130
CAS No.:908115-27-5
- SNX-2112
Catalog No.:BCC2132
CAS No.:908112-43-6
- 6,8-Di-O-methylcitreoisocoumarin
Catalog No.:BCN7380
CAS No.:908098-80-6
- Atosiban
Catalog No.:BCC5314
CAS No.:90779-69-4
- Musellactone
Catalog No.:BCN7183
CAS No.:907583-51-1
- (S)-4-Benzyl-2-oxazolidinone
Catalog No.:BCC8401
CAS No.:90719-32-7
- Ethyl beta-carboline-1-propionate
Catalog No.:BCN1311
CAS No.:90686-24-1
- α-helical CRF 9-41
Catalog No.:BCC5727
CAS No.:90880-23-2
- M871
Catalog No.:BCC5930
CAS No.:908844-75-7
- Broussonin E
Catalog No.:BCN4452
CAS No.:90902-21-9
- Erythrocentauric acid
Catalog No.:BCN7683
CAS No.:90921-13-4
- Cl-HIBO
Catalog No.:BCC7147
CAS No.:909400-43-7
- α-CGRP (human)
Catalog No.:BCC5962
CAS No.:90954-53-3
- W146
Catalog No.:BCC7723
CAS No.:909725-61-7
- A 83-01
Catalog No.:BCC1319
CAS No.:909910-43-6
- 8-Methoxybonducellin
Catalog No.:BCN4453
CAS No.:90996-27-3
- 2-Benzoylpyridine
Catalog No.:BCC8562
CAS No.:91-02-1
- 2,6-Bis(hydroxymethyl)-p-cresol
Catalog No.:BCC8505
CAS No.:91-04-3
- Syringol
Catalog No.:BCN3534
CAS No.:91-10-1
Aprotinin vs. tranexamic acid in isolated coronary artery bypass surgery: A multicentre observational study.[Pubmed:28221207]
Eur J Anaesthesiol. 2017 May;34(5):280-287.
BACKGROUND: Aprotinin appears to be more efficacious than lysine analogues to reduce bleeding and transfusion of blood products in high-transfusion-risk cardiac surgical patients. However, in isolated coronary artery bypass graft (CABG) surgery, the results from head-to-head trials remain less conclusive. OBJECTIVE: Our objective was to compare the efficacies and safety of Aprotinin and tranexamic acid (TXA) in patients undergoing isolated on-pump CABG. DESIGN: A multicentre before-and-after study pooling individual data from published trials and unpublished data from three other databases. SETTING: Four tertiary care teaching hospitals (Haut-Leveque Hospital in Bordeaux, Pitie-Salpetriere Hospital and Bichat-Claude Bernard Hospital in Paris, and Laennec Hospital in Nantes). PATIENTS: We included data of 2496 isolated on-pump CABG surgery patients who received either Aprotinin between November 2003 and May 2008 (n = 1267) or TXA between November 2007 and November 2013 (n = 1229). MAIN OUTCOME MEASURES: The primary outcome was total blood loss within 24 h after operation. Secondary outcomes were transfusion of blood products, reoperation for bleeding, renal replacement therapy, ICU length of stay and in-hospital mortality. RESULTS: Adjusted mean (SEM) 24-h blood loss after surgery [483 (11) vs. 634 (11) ml, P < 0.0001] and the proportion of patients requiring intraoperative blood product transfusion (32.7 vs. 46.5%, P = 0.01) were lower in Aprotinin-treated patients. No difference was observed with regard to reoperations for bleeding, renal replacement therapy and in-hospital mortality. However, patients receiving Aprotinin had a significantly shorter adjusted ICU length of stay. CONCLUSION: In patients undergoing isolated CABG, Aprotinin was more effective than TXA in reducing postoperative blood loss, and no safety concerns were identified. The benefits of Aprotinin should be considered when evaluating the risk of major blood loss and transfusion in patients scheduled for isolated CABG surgery.
S2'-subsite variations between human and mouse enzymes (plasmin, factor XIa, kallikrein) elucidate inhibition differences by tissue factor pathway inhibitor -2 domain1-wild-type, Leu17Arg-mutant and aprotinin.[Pubmed:27797450]
J Thromb Haemost. 2016 Dec;14(12):2509-2523.
Essentials Current antifibrinolytics - aminocaproic acid and tranexamic acid-can cause seizures or renal injury. KD1L17R -KT , Aprotinin and tranexamic acid were tested in a modified mouse tail-amputation model. S2'-subsite variations between human and mouse factor XIa result in vastly different inhibition profiles. KD1L17R -KT reduces blood loss and D-dimer levels in mouse with unobserved seizures or renal injury. SUMMARY: Background Using tissue factor pathway inhibitor (TFPI)-2 Kunitz domain1 (KD1), we obtained a bifunctional antifibrinolytic molecule (KD1L17R -KT ) with C-terminal lysine (kringle domain binding) and P2'-residue arginine (improved specificity towards plasmin). KD1L17R -KT strongly inhibited human plasmin (hPm), with no inhibition of human kallikrein (hKLK) or factor XIa (hXIa). Furthermore, KD1L17R -KT reduced blood loss comparable to Aprotinin in a mouse liver-laceration model of organ hemorrhage. However, effectiveness of these antifibrinolytic agents in a model of hemorrhage mimicking extremity trauma and their inhibition efficiencies for mouse enzymes (mPm, mKLK or mXIa) remain to be determined. Objective To determine potential differences in inhibition constants of various antifibrinolytic agents against mouse and human enzymes and test their effectiveness in a modified mouse tail-amputation hemorrhage model. Methods/Results Unexpectedly, mXIa was inhibited with ~ 17-fold increased affinity by Aprotinin (Ki ~ 20 nm) and with measurable affinity for KD1L17R -KT (Ki ~ 3 mum); in contrast, KD1WT -VT inhibited hXIa or mXIa with similar affinity. Compared with hPm, mPm had ~ 3-fold reduced affinity, whereas species specificity for hKLK and mKLK was comparable for each inhibitor. S2'-subsite variations largely accounted for the observed differences. KD1L17R -KT and Aprotinin were more effective than KD1WT -VT or tranexamic acid in inhibiting tPA-induced mouse plasma clot lysis. Further, KD1L17R -KT was more effective than KD1WT -VT and was comparable to Aprotinin and tranexamic acid in reducing blood loss and D-dimer levels in the mouse tail-amputation model. Conclusions Inhibitor potencies differ between antifibrinolytic agents against human and mouse enzymes. KD1L17R -KT is effective in reducing blood loss in a tail-amputation model that mimics extremity injury.
Elastase and tryptase govern TNFalpha-mediated production of active chemerin by adipocytes.[Pubmed:23227233]
PLoS One. 2012;7(12):e51072.
Chemerin is a leukocyte chemoattractant and adipokine with important immune and metabolic roles. Chemerin, secreted in an inactive form prochemerin, undergoes C-terminal proteolytic cleavage to generate active chemerin, a ligand for the chemokine-like receptor-1 (CMKLR1). We previously identified that adipocytes secrete and activate chemerin. Following treatment with the obesity-associated inflammatory mediator TNFalpha, unknown adipocyte mechanisms are altered resulting in an increased ratio of active to total chemerin production. Based on these findings we hypothesized adipocytes produce proteases capable of modifying chemerin and its ability to activate CMKRL1. 3T3-L1 adipocytes expressed mRNA of immunocyte and fibrinolytic proteases known to activate chemerin in vitro. Following treatment with a general protease inhibitor cocktail (PIC), the TNFalpha-stimulated increase in apparent active chemerin concentration in adipocyte media was amplified 10-fold, as measured by CMKLR1 activation. When the components of the PIC were investigated individually, Aprotinin, a serine protease inhibitor, blocked 90% of the TNFalpha-associated increase in active chemerin. The serine proteases, elastase and tryptase were elevated in adipocyte media following treatment with TNFalpha and their targeted neutralization recapitulated the Aprotinin-mediated effects. In contrast, bestatin, an aminopeptidase inhibitor, further elevated the TNFalpha-associated increase in active chemerin. Our results support that adipocytes regulate chemerin by serine protease-mediated activation pathways and aminopeptidase deactivation pathways. Following TNFalpha treatment, increased elastase and tryptase modify the balance between activation and deactivation, elevating active chemerin concentration in adipocyte media and subsequent CMKLR1 activation.
Plasmin inhibitors prevent leukocyte accumulation and remodeling events in the postischemic microvasculature.[Pubmed:21364954]
PLoS One. 2011 Feb 22;6(2):e17229.
Clinical trials revealed beneficial effects of the broad-spectrum serine protease inhibitor Aprotinin on the prevention of ischemia-reperfusion (I/R) injury. The underlying mechanisms remained largely unclear. Using in vivo microscopy on the cremaster muscle of male C57BL/6 mice, Aprotinin as well as inhibitors of the serine protease plasmin including tranexamic acid and epsilon-aminocaproic acid were found to significantly diminish I/R-elicited intravascular firm adherence and (subsequent) transmigration of neutrophils. Remodeling of collagen IV within the postischemic perivenular basement membrane was almost completely abrogated in animals treated with plasmin inhibitors or Aprotinin. In separate experiments, incubation with plasmin did not directly activate neutrophils. Extravascular, but not intravascular administration of plasmin caused a dose-dependent increase in numbers of firmly adherent and transmigrated neutrophils. Blockade of mast cell activation as well as inhibition of leukotriene synthesis or antagonism of the platelet-activating-factor receptor significantly reduced plasmin-dependent neutrophil responses. In conclusion, our data suggest that extravasated plasmin(ogen) mediates neutrophil recruitment in vivo via activation of perivascular mast cells and secondary generation of lipid mediators. Aprotinin as well as the plasmin inhibitors tranexamic acid and epsilon-aminocaproic acid interfere with this inflammatory cascade and effectively prevent postischemic neutrophil responses as well as remodeling events within the vessel wall.


