ApiinCAS# 26544-34-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26544-34-3 | SDF | Download SDF |
| PubChem ID | 5280746 | Appearance | White-yellowish powder |
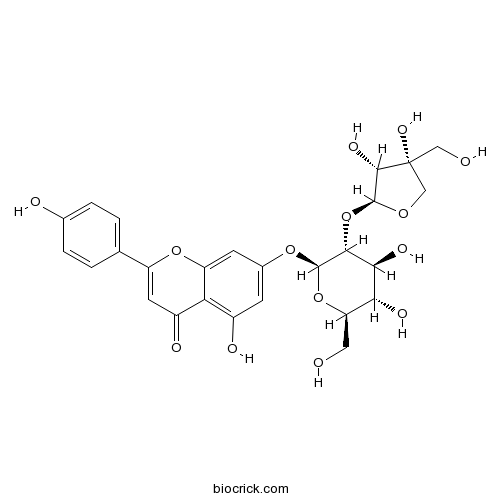
| Formula | C26H28O14 | M.Wt | 564.49 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Apigenin 7-apiosylglucoside; 4',5,7-Trihydroxyflavone 7-apiosylglucoside | ||
| Solubility | Soluble in ethanol and methan | ||
| Chemical Name | 7-[(2S,3R,4S,5S,6R)-3-[(2S,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-5-hydroxy-2-(4-hydroxyphenyl)chromen-4-one | ||
| SMILES | C1C(C(C(O1)OC2C(C(C(OC2OC3=CC(=C4C(=C3)OC(=CC4=O)C5=CC=C(C=C5)O)O)CO)O)O)O)(CO)O | ||
| Standard InChIKey | NTDLXWMIWOECHG-YRCFQSNFSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Apiin as a reducing agent, used in biological synthesis of silver and gold nanoparticles. Apiin has anti-inflammatory properties, it has inhibitory activity in-vitro on iNOS expression and nitrite production when added before LPS stimulation in the medium of J774.A1 cells. |
| Targets | NOS |
| In vitro | An extract of Apium graveolens var. dulce leaves: structure of the major constituent, apiin, and its anti-inflammatory properties.[Pubmed: 17637182]J Pharm Pharmacol. 2007 Jun;59(6):891-7.Flavonoids, natural compounds widely distributed in the plant kingdom, are reported to affect the inflammatory process and to possess anti-inflammatory as well as immunomodulatory activity in-vitro and in-vivo.
Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activities.[Pubmed: 25845643]Pharm Biol. 2016;54(1):174-9.This is the first study on the phytochemistry, antioxidant, anticholinesterase, and antibacterial activities of Sedum caeruleum L. (Crassulaceae).
The objective of this study is to isolate the secondary metabolites and determine the antioxidant, anticholinesterase, and antibacterial activities of S. caeruleum.
|
| In vivo | Bioavailability of apigenin from apiin-rich parsley in humans.[Pubmed: 16407641]Ann Nutr Metab. 2006;50(3):167-72.Absorption and excretion of apigenin after the ingestion of Apiin-rich food, i.e. parsley, was tested.
|
| Structure Identification | Colloids Surf B Biointerfaces. 2009 Jan 1;68(1):55-60.Biological synthesis of silver and gold nanoparticles using apiin as reducing agent.[Pubmed: 18977643]We report a novel strategy for the biological synthesis of anisotropic gold and quasi-spherical silver nanoparticles by using Apiin as the reducing and stabilizing agent.
|

Apiin Dilution Calculator

Apiin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7715 mL | 8.8576 mL | 17.7151 mL | 35.4302 mL | 44.2878 mL |
| 5 mM | 0.3543 mL | 1.7715 mL | 3.543 mL | 7.086 mL | 8.8576 mL |
| 10 mM | 0.1772 mL | 0.8858 mL | 1.7715 mL | 3.543 mL | 4.4288 mL |
| 50 mM | 0.0354 mL | 0.1772 mL | 0.3543 mL | 0.7086 mL | 0.8858 mL |
| 100 mM | 0.0177 mL | 0.0886 mL | 0.1772 mL | 0.3543 mL | 0.4429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Hinokinin
Catalog No.:BCN3227
CAS No.:26543-89-5
- Ryuvidine
Catalog No.:BCC7432
CAS No.:265312-55-8
- GW 7647
Catalog No.:BCC7150
CAS No.:265129-71-3
- Fosaprepitant dimeglumine salt
Catalog No.:BCC4954
CAS No.:265121-04-8
- Taiwanhomoflavone A
Catalog No.:BCN6853
CAS No.:265120-00-1
- N-[Bis(methylthio)methylene]- p-toluenesulfonamide
Catalog No.:BCC9069
CAS No.:2651-15-2
- Methyleugenolglycol
Catalog No.:BCN6562
CAS No.:26509-45-5
- Z-Gln-OH
Catalog No.:BCC2783
CAS No.:2650-64-8
- Nandrolone laurate
Catalog No.:BCC9088
CAS No.:26490-31-3
- Clovanediol diacetate
Catalog No.:BCN5144
CAS No.:2649-68-5
- Clovanediol
Catalog No.:BCN5143
CAS No.:2649-64-1
- Cyclo(D-Phe-L-Pro)
Catalog No.:BCN4011
CAS No.:26488-24-4
- 8-Phenyloctanoic acid
Catalog No.:BCC8790
CAS No.:26547-51-3
- 2,3-Dihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN5145
CAS No.:26563-68-8
- 3-Hydroxycatalponol
Catalog No.:BCN5146
CAS No.:265644-24-4
- Z-D-Glu-OMe
Catalog No.:BCC2773
CAS No.:26566-11-0
- Alisol C monoacetate
Catalog No.:BCN2345
CAS No.:26575-93-9
- Alisol B 23-acetate
Catalog No.:BCN1243
CAS No.:26575-95-1
- 5-Acetoacetlamino benzimdazolone
Catalog No.:BCC8725
CAS No.:26576-46-5
- Harmalacidine
Catalog No.:BCN8033
CAS No.:26579-69-1
- Dehydrocrenatine
Catalog No.:BCN5147
CAS No.:26585-13-7
- Crenatine
Catalog No.:BCN5148
CAS No.:26585-14-8
- SCH 202676 hydrobromide
Catalog No.:BCC7049
CAS No.:265980-25-4
- Z-D-Ala-OH
Catalog No.:BCC3059
CAS No.:26607-51-2
Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activities.[Pubmed:25845643]
Pharm Biol. 2016;54(1):174-9.
CONTEXT: This is the first study on the phytochemistry, antioxidant, anticholinesterase, and antibacterial activities of Sedum caeruleum L. (Crassulaceae). OBJECTIVE: The objective of this study is to isolate the secondary metabolites and determine the antioxidant, anticholinesterase, and antibacterial activities of S. caeruleum. MATERIALS AND METHODS: Six compounds (1-6) were isolated from the extracts of S. caeruleum and elucidated using UV, 1D-, 2D-NMR, and MS techniques. Antioxidant activity was investigated using DPPH(*), CUPRAC, and ferrous-ions chelating assays. Anticholinesterase activity was determined against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes using the Ellman method. Antibacterial activity was performed according to disc diffusion and minimum inhibitory concentration (MIC) methods. RESULTS: Isolated compounds were elucidated as ursolic acid (1), daucosterol (2), beta-sitosterol-3-O-beta-D-galactopyranoside (3), apigenin (4), apigetrin (5), and Apiin (6). The butanol extract exhibited highest antioxidant activity in all tests (IC50 value: 28.35 +/- 1.22 microg/mL in DPPH assay, IC50 value: 40.83 +/- 2.24 microg/L in metal chelating activity, and IC50 value: 23.52 +/- 0.44 microg/L in CUPRAC), and the highest BChE inhibitory activity (IC50 value: 36.89 +/- 0.15 microg/L). Moreover, the chloroform extract mildly inhibited (MIC value: 80 microg/mL) the growth of all the tested bacterial strains. DISCUSSION AND CONCLUSION: Ursolic acid (1), daucosterol (2), beta-sitosterol-3-O-beta-D-galactopyranoside (3), apigenin (4), apigetrin (5), and Apiin (6) were isolated from Sedum caeruleum for the first time. In addition, a correlation was observed between antioxidant and anticholinesterase activities of bioactive ingredients of this plant.
Biological synthesis of silver and gold nanoparticles using apiin as reducing agent.[Pubmed:18977643]
Colloids Surf B Biointerfaces. 2009 Jan 1;68(1):55-60.
We report a novel strategy for the biological synthesis of anisotropic gold and quasi-spherical silver nanoparticles by using Apiin as the reducing and stabilizing agent. The size and shape of the nanoparticles can be controlled by varying the ratio of metal salts to Apiin compound in the reaction medium. The resultant nanoparticles were characterized by UV-vis-NIR, transmission electron microscopy (TEM), FT-IR spectroscopy, X-ray diffraction (XRD) and thermogravimetric analysis (TGA). The interaction between nanoparticles with carbonyl group of Apiin compound was confirmed by using FT-IR analysis. TEM photograph confirming the average size of the gold and silver nanoparticles were found to be at 21 and 39 nm. The NIR absorption of the gold nanotriangles is expected to be of application in hyperthermia of cancer cells and in IR-absorbing optical coatings.
An extract of Apium graveolens var. dulce leaves: structure of the major constituent, apiin, and its anti-inflammatory properties.[Pubmed:17637182]
J Pharm Pharmacol. 2007 Jun;59(6):891-7.
Flavonoids, natural compounds widely distributed in the plant kingdom, are reported to affect the inflammatory process and to possess anti-inflammatory as well as immunomodulatory activity in-vitro and in-vivo. Since nitric oxide (NO) produced by inducible nitric oxide synthase (iNOS) is one of the inflammatory mediators, the effects of the ethanol/water (1:1) extract of the leaves of Apium graveolens var. dulce (celery) on iNOS expression and NO production in the J774.A1 macrophage cell line stimulated for 24 h with Escherichia coli lipopolysaccharide (LPS) were evaluated. The extract of A. graveolens var. dulce contained Apiin as the major constituent (1.12%, w/w, of the extract). The extract and Apiin showed significant inhibitory activity on nitrite (NO) production in-vitro (IC50 0.073 and 0.08 mg mL(-1) for the extract and Apiin, respectively) and iNOS expression (IC50 0.095 and 0.049 mg mL(-1) for the extract and Apiin, respectively) in LPS-activated J774.A1 cells. The croton-oil ear test on mice showed that the extract exerted anti-inflammatory activity in-vivo (ID50 730 microg cm(-2)), with a potency seven-times lower than that of indometacin (ID50 93 microg cm(-2)), the non-steroidal anti-inflammatory drug used as reference. Our results clearly indicated the inhibitory activity of the extract and Apiin in-vitro on iNOS expression and nitrite production when added before LPS stimulation in the medium of J774.A1 cells. The anti-inflammatory properties of the extract demonstrated in-vivo might have been due to reduction of iNOS enzyme expression.
Bioavailability of apigenin from apiin-rich parsley in humans.[Pubmed:16407641]
Ann Nutr Metab. 2006;50(3):167-72.
AIM: Absorption and excretion of apigenin after the ingestion of Apiin-rich food, i.e. parsley, was tested. METHODS: Eleven healthy subjects (5 women, 6 men) in the age range of 23-41 years and with an average body mass index of 23.9 +/- 4.1 kg/m2 took part in this study. After an apigenin- and luteolin-free diet, a single oral bolus of 2 g blanched parsley (corresponding to 65.8 +/- 15.5 micromol apigenin) per kilogram body weight was consumed. Blood samples were taken at 0, 4, 6, 7, 8, 9, 10, 11 and 28 h after parsley consumption and 24-hour urine samples were collected. Apigenin was analyzed in plasma, urine and red blood cells by means of HPLC-ECD. RESULTS: On average, a maximum apigenin plasma concentration of 127 +/- 81 nmol/l was reached after 7.2 +/- 1.3 h with a high range of variation between subjects. For all participants, plasma apigenin concentration rose after bolus ingestion and fell within 28 h under the detection limit (2.3 nmol/l). The average apigenin content in 24-hour urine was 144 +/- 110 nmol/24 h corresponding to 0.22 +/- 0.16% of the ingested dose. The flavone could be detected in red blood cells without showing dose-response characteristics. CONCLUSIONS: A small portion of apigenin provided by food reaches the human circulation and, therefore, may reveal biological effects.


