AntiarolCAS# 642-71-7 |
Quality Control & MSDS
Number of papers citing our products

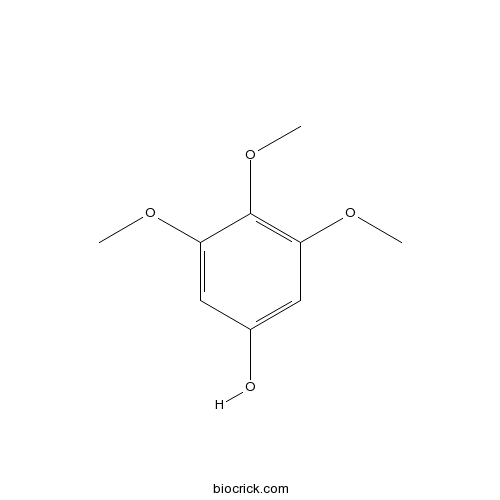
Chemical structure

3D structure
| Cas No. | 642-71-7 | SDF | Download SDF |
| PubChem ID | 69505 | Appearance | Powder |
| Formula | C9H12O4 | M.Wt | 184.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | H2O : 2 mg/mL (10.86 mM; Need ultrasonic) | ||
| Chemical Name | 3,4,5-trimethoxyphenol | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)O | ||
| Standard InChIKey | VTCDZPUMZAZMSB-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antiarol is a member of aromatic phenols and exhibits moderate DPPH free radical scavenging activity. |
| Structure Identification | Australian Journal of Chemistry, 1973 , 26 (11) :2459A revision of the structures proposed for the melicope extractives, melicopol and methylmelicopol[Reference: WebLink]2, 4, 6-Trihydroxybenzoic acid was converted by partial methylation, formylation, and then reduction into methyl 2-hydroxy-3-hydroxymethyl-4, 6-dimethoxybenzoate (5) which proved different from dimethyldegeranylmelicopol. Methyl 6-geranyloxy-2-hydroxy-3-hydroxymethyl-4-methoxybenzoate (4), its neryl analogue (7), and methyl 4-geranyloxy-2-hydroxy-3-hydroxymethyl-6-methoxybenzoate (21) were prepared similarly but all were different from methylmelicopol. From a re-investigation of earlier work, melicopol and methylmelicopol were assigned new structures, 6'-geranyloxy-2, 2', 4'-tri-hydroxy-3'-methoxyacetophenone (31) and its 4'-methyl ether derivative (24), respectively. Dimethyldegeranylmelicopol, 2, 6'-dihydroxy-2', 3', 4'-trimethoxyacetophenone (25), was prepared from Antiarol (28) and acetoxyacetonitrile. |

Antiarol Dilution Calculator

Antiarol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4289 mL | 27.1444 mL | 54.2888 mL | 108.5776 mL | 135.722 mL |
| 5 mM | 1.0858 mL | 5.4289 mL | 10.8578 mL | 21.7155 mL | 27.1444 mL |
| 10 mM | 0.5429 mL | 2.7144 mL | 5.4289 mL | 10.8578 mL | 13.5722 mL |
| 50 mM | 0.1086 mL | 0.5429 mL | 1.0858 mL | 2.1716 mL | 2.7144 mL |
| 100 mM | 0.0543 mL | 0.2714 mL | 0.5429 mL | 1.0858 mL | 1.3572 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-Quebrachitol
Catalog No.:BCN2727
CAS No.:642-38-6
- Alstonine
Catalog No.:BCN4606
CAS No.:642-18-2
- Akuammigine
Catalog No.:BCN4607
CAS No.:642-17-1
- Alloimperatorin
Catalog No.:BCC8116
CAS No.:642-05-7
- Barbinervic acid
Catalog No.:BCN4061
CAS No.:64199-78-6
- Senaetnine
Catalog No.:BCN2127
CAS No.:64191-69-1
- Zanthobungeanine
Catalog No.:BCN6685
CAS No.:64190-94-9
- Z-Hyp-Ome
Catalog No.:BCC3258
CAS No.:64187-48-0
- trans-2,3-Dihydro-3-hydroxyeuparin
Catalog No.:BCN6922
CAS No.:64185-57-5
- 30-Oxolupeol
Catalog No.:BCN6673
CAS No.:64181-07-3
- 5-Acetyl-2-(1-hydroxy-1-methylethyl)benzofuran
Catalog No.:BCN7488
CAS No.:64165-99-7
- Echinoynethiophene A
Catalog No.:BCN4183
CAS No.:64165-98-6
- CGP 12177 hydrochloride
Catalog No.:BCC6949
CAS No.:64208-32-8
- Thalirugidine
Catalog No.:BCN7706
CAS No.:64215-95-8
- Atracurium oxalate
Catalog No.:BCC8837
CAS No.:64228-78-0
- L189
Catalog No.:BCC7707
CAS No.:64232-83-3
- Taraxasterol acetate
Catalog No.:BCN4184
CAS No.:6426-43-3
- Glutinol acetate
Catalog No.:BCN6675
CAS No.:6426-44-4
- Boc-N-Me-Tyr(Bzl)-OH
Catalog No.:BCC3356
CAS No.:64263-81-6
- Kielcorin
Catalog No.:BCN7637
CAS No.:64280-48-4
- Tetrahydropapaverine HCl
Catalog No.:BCC5321
CAS No.:6429-04-5
- Acetagastrodin
Catalog No.:BCN8155
CAS No.:64291-41-4
- Galactopinitol A
Catalog No.:BCC8926
CAS No.:64290-91-1
- Dracorhodin
Catalog No.:BCC9226
CAS No.:643-56-1
Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity.[Pubmed:30081608]
Molecules. 2018 Aug 5;23(8). pii: molecules23081952.
The sterols beta-sitostenone (1), stigmast-4,6,8(14),22-tetraen-3-one (2), beta-sitosterol (3) and stigmasterol (4), the aromatic derivatives Antiarol (5) and gentisic acid (6), the phenylpropanes coniferyl alcohol (7), epoxyconiferyl alcohol (8) and ferulic acid (9), the apocarotenoid vomifoliol (10), the flavonoids naringenin (11), 7,4'-dimethoxytaxifolin (7,4'-dimethoxydihydroquercetin, 12), aromadendrin (13), kaempferol (14), taxifolin (dihydroquercetin, 15), prunin (naringenin-7-O-beta-d-glucoside, 16), populnin (kaempferol-7-O-beta-d-glucoside, 17) and senecin (aromadendrin-7-O-beta-d-glucoside, 18) and the lignans kobusin (19) and pinoresinol (20), were isolated from the dried bark of Cochlospermum vitifolium Spreng (Cochlospermaceae), a Mexican medicinal plant used to treat jaundice, liver ailments and hepatitis C. Fourteen of these compounds were isolated for the first time from this plant and from the Cochlospermum genus. Compounds 3(-)4, 6(-)7, 9(-)11, 13(-)17 and 20 have previously exhibited diverse beneficial liver activities. The presence of these compounds in C. vitifolium correlates with the use of this Mexican medicinal plant.
Antiarol cinnamate and africanoside, a cinnamoyl triterpene and a hydroperoxy-cardenolide from the stem bark of Antiaris africana.[Pubmed:20533166]
Planta Med. 2010 Oct;76(15):1717-23.
From the methanol extract of the stem bark of the African tree Antiaris africana Engler, two new bioactive metabolites were isolated, namely, the alpha-amyrin derivative 1beta,11alpha-dihydroxy-3beta-cinnamoyl-alpha-amyrin (Antiarol cinnamate, 1) and a cardiac glycoside, 3beta-O-(alpha-L-rhamnopyranosyl)-14beta-hydroperoxy-5beta-hydroxy-19-oxo-17beta- card-20(22)-enolide (africanoside, 2a), together with the known compounds beta-amyrin and its acetate, beta-sitosterol and its 3-O-beta-D-glucopyranoside, friedelin, ursolic and oleanolic acid, 19-norperiplogenin, strophanthidol, strophanthidinic acid, periplogenin (3a), 3-epiperiplogenin, strophanthidin (3b) and 3,3'-dimethoxy-4'-O-beta-D-xylopyronosyl-ellagic acid. Their structures were established on the basis of their spectroscopic data and by chemical methods, while 3a was additionally confirmed by X-ray crystal structure analysis. The aglycone moiety possessing a hydroperoxy group was found for the first time in cardenolides. Compounds 1 and 2a showed no activity against bacteria, fungi, and microalgae; however, the crude extract exhibited a high toxicity against Artemia salina and a selective antitumor activity against human tumor cell lines. Africanoside (2a) effected a concentration-dependent inhibition of tumor cell growth with a mean IC(50) value of 5.3 nM.
Indonesian Medicinal Plants. XIV. Characterization of 3'-O-Caffeoylsweroside, a new secoiridoid glucoside, and kelampayosides A and B, two new phenolic apioglucosides, from the bark of Anthocephalus chinensis (Rubiaceae).[Pubmed:8814946]
Chem Pharm Bull (Tokyo). 1996 Jun;44(6):1162-7.
A new secoiridoid glucoside named 3'-O-caffeoylsweroside (1), and two new phenolic apioglucosides, named kelampayoside A (4) and kelampayoside B (6), together with eleven known compounds (five iridoids and six alkaloids), were isolated from the bark of Anthocephalus chinensis (Rubiaceae), an Indonesian medicinal plant from Sumatra Island, Indonesia. The chemical structures of 1, 4 and 6 have been elucidated respectively as 3'-O-caffeoylsweroside (1), Antiarol 1-O-beta-D-apiofuranosyl (1 --> 6)-beta-D-glucopyranoside (4), and Antiarol 1-O-beta-D-5"-O-caffeoylapiofuransoyl (1 --> 6)-beta-D-glucopyranoside (6) on the bases of their chemical and physiochemical properties. Among fourteen constituents characterized, cadambine (13), one of the major indole alkaloid constituents of A. chinensis, was shown to exhibit moderate growth-inhibitory activity against the malarial parasite Plasmodium falciparum (a chloroquine-resistant K1 strain) cultured in human erythrocytes.


