Alpha-SantoninCAS# 481-06-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

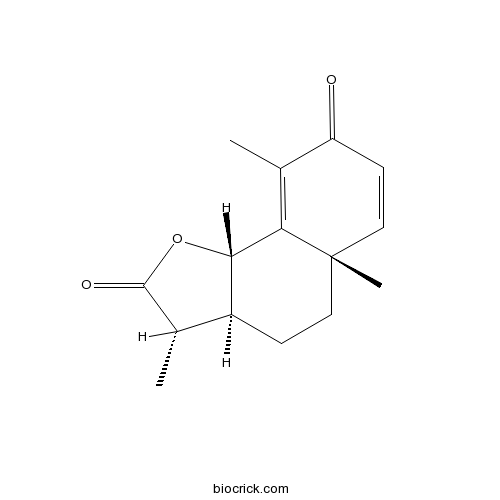
| Cas No. | 481-06-1 | SDF | Download SDF |
| PubChem ID | 221071 | Appearance | White crystalline |
| Formula | C15H18O3 | M.Wt | 246.30 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Semenen | ||
| Solubility | DMSO : 62.5 mg/mL (253.76 mM; Need ultrasonic) | ||
| Chemical Name | (3S,3aS,5aS,9bS)-3,5a,9-trimethyl-3a,4,5,9b-tetrahydro-3H-benzo[g][1]benzofuran-2,8-dione | ||
| SMILES | CC1C2CCC3(C=CC(=O)C(=C3C2OC1=O)C)C | ||
| Standard InChIKey | XJHDMGJURBVLLE-BOCCBSBMSA-N | ||
| Standard InChI | InChI=1S/C15H18O3/c1-8-10-4-6-15(3)7-5-11(16)9(2)12(15)13(10)18-14(8)17/h5,7-8,10,13H,4,6H2,1-3H3/t8-,10-,13-,15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Alpha-Santonin is a well known anthelminthic agent, it also has immunosuppressant, cytotoxic, and anti-parasitic activities. |
| Targets | Immunology & Inflammation related |
| In vitro | Diminutive effect on T and B-cell proliferation of non-cytotoxic α-santonin derived 1,2,3-triazoles: a report.[Pubmed: 23314050 ]Eur J Med Chem. 2013 Feb;60:365-75.
|
| Structure Identification | Acta Crystallogr Sect E Struct Rep Online. 2012 Jul 1;68(Pt 7):o2112.3,5a,9-Trimethyl-8-(2-phenylhydrazin-1-ylidene)-4,5,5a,9b-tetrahydro-3aH,8H-naphtho[1,2-b]furan-2(3H)-one.[Pubmed: 22798789 ]The title compound, C(21)H(24)N(2)O(2), is a phenyl hydrazine derivative of the well known anthelminthic agent Alpha-Santonin, which is composed of three fused rings (benzodieneone, cyclo-hexane and γ-lactone). The cyclo-hexa-dienone ring adopts a boat conformation, the cyclo-hexane ring is in a chair conformation and the trans-fused γ-lactone ring adopts a C-envelope conformation. In the crystal, mol-ecules are linked by N-H⋯O and C-H⋯O hydrogen bonds, forming chains along the a axis. Z Naturforsch C. 2000 Sep-Oct;55(9-10):713-7.Biotransformation of two cytotoxic terpenes, alpha-santonin and sclareol by Botrytis cinerea.[Pubmed: 11098821]Two cytotoxic terpenes, Alpha-Santonin (1) and sclareol (3) were biotransformed by a plant pathogenic fungus Botrytis cinerea to produce oxidized metabolites in high yields. J Chromatogr. 1992 Feb 28;593(1-2):209-15.Use of high-performance liquid chromatographic peak deconvolution and peak labelling to identify antiparasitic components in plant extracts.[Pubmed: 1639905]Artemisia absynthium L. is a commonly used medicinal plant for parasitic diseases all over the world. |

Alpha-Santonin Dilution Calculator

Alpha-Santonin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Edoxaban tosylate
Catalog No.:BCC1544
CAS No.:480449-71-6
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- Lucialdehyde B
Catalog No.:BCN2450
CAS No.:480439-84-7
- TFB-TBOA
Catalog No.:BCC5919
CAS No.:480439-73-4
- Carbenicillin, Disodium Salt
Catalog No.:BCC1200
CAS No.:4800-94-6
- Benzofuroxan
Catalog No.:BCC8852
CAS No.:480-96-6
- Dicrotaline
Catalog No.:BCN2079
CAS No.:480-87-5
- Retusine
Catalog No.:BCN2123
CAS No.:480-86-4
- Retronecine
Catalog No.:BCN2034
CAS No.:480-85-3
- Echinatine
Catalog No.:BCN1968
CAS No.:480-83-1
- Indicine
Catalog No.:BCN1995
CAS No.:480-82-0
- Seneciphylline
Catalog No.:BCN5563
CAS No.:480-81-9
- alpha-Spinasterol
Catalog No.:BCN5564
CAS No.:481-18-5
- Epiandrosterone
Catalog No.:BCC4481
CAS No.:481-29-8
- Ecgonine
Catalog No.:BCN1907
CAS No.:481-37-8
- Juglone
Catalog No.:BCN2639
CAS No.:481-39-0
- Plumbagin
Catalog No.:BCN2586
CAS No.:481-42-5
- Ginkgetin
Catalog No.:BCN2319
CAS No.:481-46-9
- Cepharanthine
Catalog No.:BCN5393
CAS No.:481-49-2
- Tangeretin
Catalog No.:BCN2386
CAS No.:481-53-8
- Aloeemodin
Catalog No.:BCN5565
CAS No.:481-72-1
- Citreorosein
Catalog No.:BCN5566
CAS No.:481-73-2
- Chrysophanol
Catalog No.:BCN5567
CAS No.:481-74-3
- Estriol 3-sulfate
Catalog No.:BCN2236
CAS No.:481-95-8
Diminutive effect on T and B-cell proliferation of non-cytotoxic alpha-santonin derived 1,2,3-triazoles: a report.[Pubmed:23314050]
Eur J Med Chem. 2013 Feb;60:365-75.
Alpha-Santonin derived new series of 1,2,3-triazoles synthesized through Azide-Alkyne Huisgen 1,3-dipolar cycloaddition reaction between substituted aryl azide and a propargylated alpha-desmotrosantonin were bio-evaluated for their diminutive effect on ConA induced T-cell and LPS induced B-cell proliferation. Interestingly, most of the synthesized compounds showed better immunosuppressant activity than Alpha-Santonin. Triazole derivatives 9, 10, 17, 18, 29, and 30 displayed significant diminutive effect on cell proliferation. Compounds 12 and 13 were found selective against ConA T-cell proliferation exhibiting >90% inhibition at 1 x 10(-6) M concentration. The present study resulted in identification of several triazole derivatives as effective immunosuppressive agents.
Biotransformation of two cytotoxic terpenes, alpha-santonin and sclareol by Botrytis cinerea.[Pubmed:11098821]
Z Naturforsch C. 2000 Sep-Oct;55(9-10):713-7.
Two cytotoxic terpenes, Alpha-Santonin (1) and sclareol (3) were biotransformed by a plant pathogenic fungus Botrytis cinerea to produce oxidized metabolites in high yields. Alpha-Santonin (1) on fermentation with the fungus for ten days afforded a hydroxylated metabolite identified as 11beta-hydroxy-Alpha-Santonin (2) in a high yield (83%), while sclareol (3) was metabolized to epoxysclareol (4) (64%) and a new compound 8-deoxy-14,15-dihydro-15-chloro-14-hydroxy-8,9-dehydrosclareol (5) (7%), representing a rare example of microbial halogenation.
Use of high-performance liquid chromatographic peak deconvolution and peak labelling to identify antiparasitic components in plant extracts.[Pubmed:1639905]
J Chromatogr. 1992 Feb 28;593(1-2):209-15.
Artemisia absynthium L. is a commonly used medicinal plant for parasitic diseases all over the world. By means of high-performance liquid chromatography with diode-array detection and the PU6100 solvent optimization system, two sesquiterpene lactones, Alpha-Santonin and ketopelenolid-A, were tentatively identified in methanolic extracts of this plant. Alpha-Santonin is a well known antiparasitic compound and could be one of the active principles of this plant species. Reconstructed spectra are potentially useful in scanning a complex chromatogram for pharmacologically active compounds.
3,5a,9-Trimethyl-8-(2-phenylhydrazin-1-ylidene)-4,5,5a,9b-tetrahydro-3aH,8H-napht ho[1,2-b]furan-2(3H)-one.[Pubmed:22798789]
Acta Crystallogr Sect E Struct Rep Online. 2012 Jul 1;68(Pt 7):o2112.
The title compound, C(21)H(24)N(2)O(2), is a phenyl hydrazine derivative of the well known anthelminthic agent Alpha-Santonin, which is composed of three fused rings (benzodieneone, cyclo-hexane and gamma-lactone). The cyclo-hexa-dienone ring adopts a boat conformation, the cyclo-hexane ring is in a chair conformation and the trans-fused gamma-lactone ring adopts a C-envelope conformation. In the crystal, mol-ecules are linked by N-Hcdots, three dots, centeredO and C-Hcdots, three dots, centeredO hydrogen bonds, forming chains along the a axis.


