Albanin ACAS# 73343-42-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

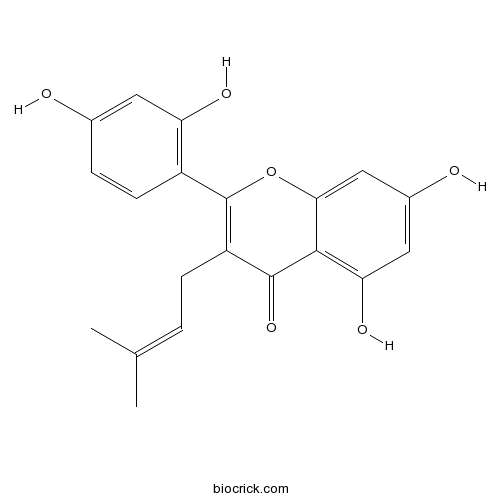
| Cas No. | 73343-42-7 | SDF | Download SDF |
| PubChem ID | 5481961 | Appearance | Yellow powder |
| Formula | C20H18O6 | M.Wt | 354.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-3-(3-methylbut-2-enyl)chromen-4-one | ||
| SMILES | CC(=CCC1=C(OC2=CC(=CC(=C2C1=O)O)O)C3=C(C=C(C=C3)O)O)C | ||
| Standard InChIKey | KEIIIPKLVSSAEI-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Albanin A shows mushroom tyrosinase inhibitory activity, the IC50 value is 463 uM. 2. In melanin formation inhibition on B16 melanoma cells, the IC50 of albanin A is 40.1 uM. |
| Targets | Tyrosinase |

Albanin A Dilution Calculator

Albanin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8217 mL | 14.1084 mL | 28.2167 mL | 56.4334 mL | 70.5418 mL |
| 5 mM | 0.5643 mL | 2.8217 mL | 5.6433 mL | 11.2867 mL | 14.1084 mL |
| 10 mM | 0.2822 mL | 1.4108 mL | 2.8217 mL | 5.6433 mL | 7.0542 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5643 mL | 1.1287 mL | 1.4108 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5643 mL | 0.7054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Macbecin I
Catalog No.:BCC7551
CAS No.:73341-72-7
- 3-Hydroxy-9,10-Dimethoxypterocarpan
Catalog No.:BCC8101
CAS No.:73340-41-7
- Methylnissolin
Catalog No.:BCN1368
CAS No.:733-40-4
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
- Deoxynojirimycin hydrochloride
Catalog No.:BCN2626
CAS No.:73285-50-4
- 1,4-Dihydro-1,2-dimethyl-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCN1369
CAS No.:73281-83-1
- 5-O-Caffeoylshikimic acid
Catalog No.:BCN7929
CAS No.:73263-62-4
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
- Methylnaltrexone Bromide
Catalog No.:BCC1740
CAS No.:73232-52-7
- Florfenicol
Catalog No.:BCC8984
CAS No.:73231-34-2
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- 9-O-Acetyl-4,4'-di-O-methyllariciresinol
Catalog No.:BCN1367
CAS No.:73354-15-1
- Niclosamide monohydrate
Catalog No.:BCC5212
CAS No.:73360-56-2
- SCH 50911
Catalog No.:BCC5692
CAS No.:733717-87-8
- SB 706375
Catalog No.:BCC6256
CAS No.:733734-61-7
- AR 231453
Catalog No.:BCC5143
CAS No.:733750-99-7
- Norandrostenedione
Catalog No.:BCC9103
CAS No.:734-32-7
- Dehydrobruceine A
Catalog No.:BCN7620
CAS No.:73435-47-9
- 1-Deoxymannojirimycin hydrochloride
Catalog No.:BCC6995
CAS No.:73465-43-7
- Tetrachyrin
Catalog No.:BCN4776
CAS No.:73483-88-2
- 7-Acetyllycopsamine
Catalog No.:BCN2000
CAS No.:73544-48-6
- cis-ACBD
Catalog No.:BCC6587
CAS No.:73550-55-7
- Mevastatin
Catalog No.:BCN2568
CAS No.:73573-88-3
Isoprenylated Flavonoids from Roots of Artocarpus styracfolius.[Pubmed:30508347]
Nat Prod Commun. 2016 Dec;11(12):1843-1846.
Seven isoprenylated flavonoids were isolated from Artocarpus styracifolius, including one new triisoprenylated flavone, styracifolin D (1,) and six known ones, artocarpone B (2), kuwanon C (3), 6-C-prenyl luteolin (4), Albanin A (5), 2,4,2',4'-tetrahydroxy-3'-(3-methyl-2-butenyl)-chalcone (6), and 3'-[gamma-hydroxymethyl-(E)- gamma-methylallyl]-2,4,2',4'-tetrahydroxychalcone 11'-0-coumarate (7). The structures of these compounds were determined by analysis of their spectroscopic and mass spectrometric data. Of them, 3 and 5 exhibited inhibitory effects on cathepsin K with IC(50) values of 114.6 and 7.4 muM, respectively.
Antioxidant and nitrite-scavenging capacities of phenolic compounds from sugarcane (Saccharum officinarum L.) tops.[Pubmed:25162956]
Molecules. 2014 Aug 26;19(9):13147-60.
Sugarcane tops were extracted with 50% ethanol and fractionated by petroleum ether, ethyl acetate (EtOAc), and n-butyl alcohol successively. Eight phenolic compounds in EtOAc extracts were purified through silica gel and Sephadex LH-20 column chromatographies, and then identified by nuclear magnetic resonance and electrospray ionization mass spectra. The results showed that eight phenolic compounds from EtOAc extracts were identified as caffeic acid, cis-p-hydroxycinnamic acid, quercetin, apigenin, Albanin A, australone A, moracin M, and 5'-geranyl-5,7,2',4'-tetrahydroxyflavone. The antioxidant and nitrite-scavenging capacities of different solvent extracts correlated positively with their total phenolic (TP) contents. Amongst various extracts, EtOAc extracts possessed the highest TP content and presented the strongest oxygen radical absorbance capacity (ORAC), 1,1'-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging capacity, 2,2'-azobis-3-ethylbenthiaazoline-6-sulfonic acid (ABTS) radical-scavenging capacity, ferric reducing antioxidant power (FRAP) and nitrite-scavenging capacity. Thus, sugarcane tops could be promoted as a source of natural antioxidant.
[Chemical constituents from cell cultures of Morus alba].[Pubmed:23627170]
Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(24):3738-42.
The column chromatography on silica gel, Sephadex LH-20 and semi-preparative HPLC were used to separate and purify the compounds from the EtOAc extract of medium and MeOH extract of cell cultures of Morus alba. Eight compounds were isolated. Based on physico-chemical properties and spectroscopic data, their structures were identified as isobavachalcone (1), genistein (2), norartocarpetin (3), Albanin A (4), guangsangon E (5), mulberrofuran F (6), chalcomoracin (7), kuwanon J (8). Compounds 3-6 were isolated from the cell cultures of M. alba for the first time.
Artocarpus plants as a potential source of skin whitening agents.[Pubmed:21941923]
Nat Prod Commun. 2011 Sep;6(9):1397-402.
Artocarpus plants have been a focus of constant attention due to the potential for skin whitening agents. In the in vitro experiment, compounds from the Artocarpus plants, such as artocarpanone, norartocarpetin, artocarpesin, artogomezianol, andalasin, artocarbene, and chlorophorin showed tyrosinase inhibitory activity. Structure-activity investigations revealed that the 4-substituted resorcinol moiety in these compounds was responsible for their potent inhibitory activities on tyrosinase. In the in vitro assay, using B16 melanoma cells, the prenylated polyphenols isolated from Artocarpus plants, such as artocarpin, cudraflavone C, 6-prenylapigenin, kuwanon C, norartocarpin, Albanin A, cudraflavone B, and brosimone I showed potent inhibitory activity on melanin formation. Structure-activity investigations revealed that the introduction of an isoprenoid moiety to a non-isoprenoid-substituted polyphenol enhanced the inhibitory activity of melanin production in B16 melanoma cells. In the in vivo investigation, the extract of the wood of Artocarpus incisus and a representative isolated compound from it, artocarpin had a lightening effect on the skin of guinea pigs' backs. Other in vivo experiments using human volunteers have shown that water extract of Artocarpus lakoocha reduced the melanin formation in the skin of volunteers. These results indicate that the extracts of Artocarpus plants are potential sources for skin whitening agents.
Three new compounds from Morus nigra L.[Pubmed:20552479]
J Asian Nat Prod Res. 2010 Jun;12(6):431-7.
A new 2-arylbenzofuran derivative, mornigrol D (1), along with two new flavones, mornigrol G (2) and mornigrol H (3), and six known compounds, norartocarpetin (4), dihydrokaempferol (5), Albanin A (6), albanin E (7), moracin M (8), and albafuran C (9), were isolated from the barks of Morus nigra. Their structures were elucidated by spectroscopic analysis. Compounds 1 and 9 showed antioxidative activities in vitro with inhibition ratios of 98 and 99% at the concentration of 10(-4) mol/l, and of 74 and 75% at the concentration of 10(-5) mol/l. In addition, compounds 1 and 4 showed potent anti-inflammatory activities (inhibition of release of beta-glucuronidase from rat polymorphonuclear leucocytes induced by platelet activating factor) with inhibitory ratios of 65.9% (P < 0.01) and 67.7% (P < 0.01) at a concentration of 10(-5) mol/l.
New isoprenylated 2-arylbenzofurans and pancreatic lipase inhibitory constituents from Artocarpus nitidus.[Pubmed:20020453]
Chem Biodivers. 2009 Dec;6(12):2209-16.
Two new isoprenylated 2-arylbenzofurans, artonitidin A (=(2'R)-2',3'-dihydro-2'-(1-hydroxy-1-methylethyl)-5',7-bis(3-methylbut-2-en-1-yl )-2,4'-bi-1-benzofuran-6,6'-diol; and artonitidin B (=5-[6-hydroxy-7-(3-methylbut-2-en-1-yl)-1-benzofuran-2-yl]-4-(3-methylbut-2-en-1 -yl)benzene-1,3-diol; together with 14 known compounds, were isolated from the stems of Artocarpus nitidus Trec. The structures were elucidated by spectroscopic methods. Norartocarpin, cudraflavone C, brosimone I, artotonkin, Albanin A, and artopetelin M showed inhibitory effects on pancreatic lipase with IC(50) values ranging from 1.8+/-0.1 to 63.8+/-3.6 microM.
Isoprenoid-substituted flavonoids from wood of Artocarpus heterophyllus on B16 melanoma cells: cytotoxicity and structural criteria.[Pubmed:19686821]
Fitoterapia. 2010 Mar;81(2):120-3.
As a result of cytotoxicity-guided fractionation, nine flavonoids, artocarpin (1), cudraflavone C (2), 6-prenylapigenin (3), kuwanon C (4), norartocarpin (5), Albanin A (6), cudraflavone B (7), brosimone I (8) and artocarpanone (9) were identified from the methanol extract of the wood of Artocarpus heterophyllus, known commonly as Nangka in Indonesia. A structure-activity investigation of the effect of these isolated compounds (1-9) and structurally related compounds on B16 melanoma cells indicated that isoprenoid moiety substitutions in flavonoids enhance their cytotoxicity, and that the position of attachment and the number of isoprenoid-substituent moieties per molecule influence flavonoid cytotoxicity.
Inhibitory effect of isoprenoid-substituted flavonoids isolated from Artocarpus heterophyllus on melanin biosynthesis.[Pubmed:16732541]
Planta Med. 2006 Jul;72(9):847-50.
Isoprenoid-substituted flavonoids were isolated from the wood of Artocarpus heterophyllus by means of activity-guided fractionation. Artocarpin (1), cudraflavone C (2), 6-prenylapigenin (3), kuwanon C (4), norartocarpin (5) and Albanin A (6) inhibited melanin biosynthesis in B16 melanoma cells without inhibiting tyrosinase. A structure-activity investigation indicated that the presence of the isoprenoid-substituted moiety enhanced the inhibitory activity on melanin production in B16 melanoma cells.
Tyrosinase inhibitors from Artocarpus gomezianus.[Pubmed:10821057]
Planta Med. 2000 Apr;66(3):275-7.
Eight compounds including, phenyl-beta-naphthylamine (1), isocyclomorusin (2), cycloartocarpin (3), artocarpin (4), norartocarpetin (5), cudraflavone C (6), Albanin A (7), and resveratrol (8) were isolated from the roots of Artocarpus gomezianus. Compounds 5 and 8 exhibited potent tyrosinase inhibitory activity. The 1H- and 13C-NMR properties of 1, 3 and 8 were extensively studied.
Structures of three new flavone derivatives, brosimones G, H, and I, from Brosimopsis oblongifolia.[Pubmed:17262259]
Planta Med. 1989 Feb;55(1):70-2.
From the roots of BROSIMOPSIS OBLONGIFOLIA three new flavone derivatives, named brosimone G ( 3), H ( 4), and I ( 5) together with the known Albanin A ( 1) and E ( 2), were isolated. Antimicrobial activity of the compounds is also reported.


