(±)-AC 7954 hydrochlorideNon-peptide UT receptor agonist CAS# 477313-09-0 |
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 477313-09-0 | SDF | Download SDF |
| PubChem ID | 9907171 | Appearance | Powder |
| Formula | C19H21Cl2NO2 | M.Wt | 366.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 75 mM in water and to 75 mM in DMSO | ||
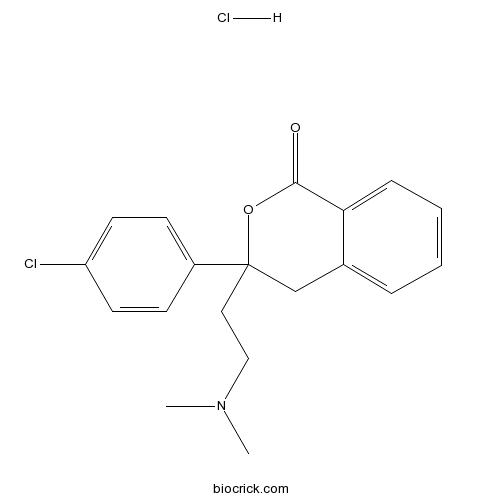
| Chemical Name | 3-(4-chlorophenyl)-3-[2-(dimethylamino)ethyl]-4H-isochromen-1-one;hydrochloride | ||
| SMILES | CN(C)CCC1(CC2=CC=CC=C2C(=O)O1)C3=CC=C(C=C3)Cl.Cl | ||
| Standard InChIKey | AJPGLOWFIBOGMH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H20ClNO2.ClH/c1-21(2)12-11-19(15-7-9-16(20)10-8-15)13-14-5-3-4-6-17(14)18(22)23-19;/h3-10H,11-13H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly selective, non-peptide urotensin-II (UT) receptor agonist that displays potent activity at human and rat UT receptors (pEC50 values are 6.5 and 6.7 respectively). |

(±)-AC 7954 hydrochloride Dilution Calculator

(±)-AC 7954 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7302 mL | 13.6508 mL | 27.3015 mL | 54.603 mL | 68.2538 mL |
| 5 mM | 0.546 mL | 2.7302 mL | 5.4603 mL | 10.9206 mL | 13.6508 mL |
| 10 mM | 0.273 mL | 1.3651 mL | 2.7302 mL | 5.4603 mL | 6.8254 mL |
| 50 mM | 0.0546 mL | 0.273 mL | 0.546 mL | 1.0921 mL | 1.3651 mL |
| 100 mM | 0.0273 mL | 0.1365 mL | 0.273 mL | 0.546 mL | 0.6825 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mangiferin
Catalog No.:BCN5535
CAS No.:4773-96-0
- Bergenin
Catalog No.:BCN5540
CAS No.:477-90-7
- Digitolutein
Catalog No.:BCN3089
CAS No.:477-86-1
- Obtusifolin
Catalog No.:BCN2537
CAS No.:477-85-0
- Damnacanthal
Catalog No.:BCN5539
CAS No.:477-84-9
- Isochondodendrine
Catalog No.:BCC9234
CAS No.:477-62-3
- Isotetrandrine
Catalog No.:BCN5538
CAS No.:477-57-6
- Podophyllotoxinone
Catalog No.:BCN8063
CAS No.:477-49-6
- Beta-Apopicropodophyllin
Catalog No.:BCC1388
CAS No.:477-52-1
- Dehydrocostus lactone
Catalog No.:BCN5536
CAS No.:477-43-0
- Samidin
Catalog No.:BCN6665
CAS No.:477-33-8
- Demecolcine
Catalog No.:BCC9223
CAS No.:477-30-5
- cis-ACPD
Catalog No.:BCC6566
CAS No.:477331-06-9
- Ophiopogonanone C
Catalog No.:BCN6620
CAS No.:477336-75-7
- Sculponeatin K
Catalog No.:BCN5537
CAS No.:477529-70-7
- Musellarin A
Catalog No.:BCN7186
CAS No.:477565-36-9
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- Tofacitinib (CP-690550,Tasocitinib)
Catalog No.:BCC2192
CAS No.:477600-75-2
- PIM-1 Inhibitor 2
Catalog No.:BCC2446
CAS No.:477845-12-8
- 3-(2-Glucosyloxy-4-methoxyphenyl)propanoic acid
Catalog No.:BCN7068
CAS No.:477873-63-5
- Neostenine
Catalog No.:BCN5541
CAS No.:477953-07-4
- Nobiletin
Catalog No.:BCN5542
CAS No.:478-01-3
- Lucidin
Catalog No.:BCC1709
CAS No.:478-08-0
- Pseudoaspidin
Catalog No.:BCN6386
CAS No.:478-28-4
Novel potent and efficacious nonpeptidic urotensin II receptor agonists.[Pubmed:16570919]
J Med Chem. 2006 Apr 6;49(7):2232-40.
Six different series of nonpeptidic urotensin II receptor agonists have been synthesized and evaluated for their agonistic activity in a cell-based assay (R-SAT). The compounds are ring-opened analogues of the isochromanone-based agonist AC-7954 with different functionalities constituting the linker between the two aromatic ring moieties. Several of the compounds are highly potent and efficacious, with N-[1-(4-chlorophenyl)-3-(dimethylamino)-propyl]-4-phenylbenzamide oxalate (5d) being the most potent. The pure enantiomers of 5d were obtained from the corresponding diastereomeric amides. It was shown by a combination of X-ray crystallography and chemical correlation that the activity resides in the S-enantiomer of 5d (pEC(50) 7.49).
Architecture of the human urotensin II receptor: comparison of the binding domains of peptide and non-peptide urotensin II agonists.[Pubmed:15801838]
J Med Chem. 2005 Apr 7;48(7):2480-92.
The human urotensin II receptor (h-UTR) is a member of the family of rhodopsin-like G-protein-coupled receptors (GPCRs) involved in the modulation of the functionality of many tissues and organs. Recently the urotensin-II (UII) neuropeptide, which is a potent vasoconstrictor in mammals and it is postulated to play a central role in cardiovascular homeostasis, has been identified as an agonist of the UII receptor. To elucidate the receptor's molecular recognition, a h-UTR model was constructed by homology modeling using the 2.6 A crystal structure of bovine rhodopsin as a template and subsequently refined by molecular dynamics simulations. The molecular recognition of h-UTR was probed by automated docking of P5U, a potent UII peptide agonist, as well as of the non-peptide compounds 1-4. We believe that this new model of the h-UTR provides the means for understanding the ligand's potency and for facilitating the design of novel and more potent UII ligands.
Discovery of the first nonpeptide agonist of the GPR14/urotensin-II receptor: 3-(4-chlorophenyl)-3-(2- (dimethylamino)ethyl)isochroman-1-one (AC-7954).[Pubmed:12408704]
J Med Chem. 2002 Nov 7;45(23):4950-3.
A functional cell-based screen identified 3-(4-chlorophenyl)-3-(2-(dimethylamino)ethyl)isochroman-1-one hydrochloride (AC-7954, 1) as a nonpeptidic agonist of the urotensin-II receptor. Racemic 1 had an EC50 of 300 nM at the human UII receptor and was highly selective. Testing of the enantiopure (+)- and (-)- 1 revealed that the UII receptor activity of racemic 1 resides primarily in (+)-1. Being a selective nonpeptidic druglike UII receptor agonist, (+)-1 will be useful as a pharmacological research tool and a potential drug lead.


