A 61603 hydrobromidePotent α1A-AR agonist CAS# 107756-30-9 |
- PHT-427
Catalog No.:BCC2554
CAS No.:1191951-57-1
- AT7867 dihydrochloride
Catalog No.:BCC1378
CAS No.:1431697-86-7
- Perifosine
Catalog No.:BCC3673
CAS No.:157716-52-4
- TIC10
Catalog No.:BCC3906
CAS No.:41276-02-2
- A-674563
Catalog No.:BCC3903
CAS No.:552325-73-2
- CCT128930
Catalog No.:BCC3904
CAS No.:885499-61-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 107756-30-9 | SDF | Download SDF |
| PubChem ID | 9865178 | Appearance | Powder |
| Formula | C14H20BrN3O3S | M.Wt | 390.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in water | ||
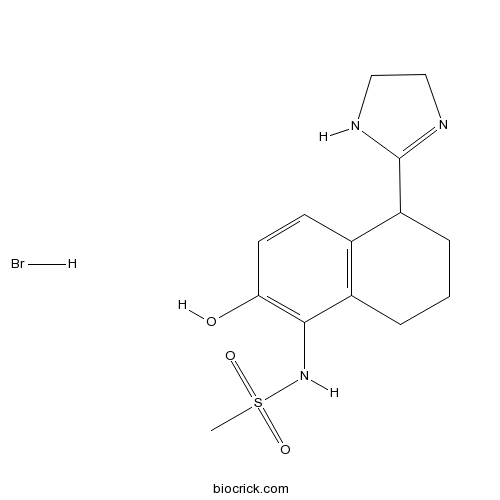
| Chemical Name | N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl]methanesulfonamide;hydrobromide | ||
| SMILES | CS(=O)(=O)NC1=C(C=CC2=C1CCCC2C3=NCCN3)O.Br | ||
| Standard InChIKey | LRFLWCZMTGTUEP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H19N3O3S.BrH/c1-21(19,20)17-13-10-3-2-4-11(14-15-7-8-16-14)9(10)5-6-12(13)18;/h5-6,11,17-18H,2-4,7-8H2,1H3,(H,15,16);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent α-adrenoceptor agonist that is at least 35-fold more potent at α1A than at α1B or α1D sites. Induces dose response increases in spontaneous Ca2+ transients in rat ventricular myocytes in vitro (EC50 = 6.9 nmol/L). Also available as part of the α1-Adrenoceptor. |

A 61603 hydrobromide Dilution Calculator

A 61603 hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5622 mL | 12.811 mL | 25.622 mL | 51.2439 mL | 64.0549 mL |
| 5 mM | 0.5124 mL | 2.5622 mL | 5.1244 mL | 10.2488 mL | 12.811 mL |
| 10 mM | 0.2562 mL | 1.2811 mL | 2.5622 mL | 5.1244 mL | 6.4055 mL |
| 50 mM | 0.0512 mL | 0.2562 mL | 0.5124 mL | 1.0249 mL | 1.2811 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2562 mL | 0.5124 mL | 0.6405 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A 61603 hydrobromide is a potent and selective agonist of α1A-adrenoceptor [1].
α1A-adrenoceptor (α1A-AR) belongs to α1- adrenergic receptors, which include three subtypes α1A, α1B and α1D and play an important role in regulating cell growth and proliferation.
A 61603 hydrobromide is a potent and selective α1A-adrenoceptor agonist. A-61603 was 35-fold more potent at α1A-AR than at α1B-AR or α1D-AR. In fibroblast cells transfected with α1A-AR, A-61603 significantly stimulated phosphoinositide hydrolysis [1]. A 61603 exhibited affinity for α1A-AR, α1B-AR and α1D-AR with pKi values of 8.05/7.52, 5.68 and 5.87, respectively. Also, A 61603 exhibited agonist activities for α1A-AR, α1B-AR and α1D-AR with pEC50 values of 8.24/7.66, 6.50 and 5.59, respectively [2]. In neonatal rat ventricular myocytes, A61603 significantly increased the frequency of Ca2+ transients with EC50 value of 6.9 nM in a dose-dependent way [3]. In mesenteric vascular bed isolated from mice, A61603 significantly increased perfusion pressure with 235-fold higher potency than phenylephrine, which suggested that α1A-AR plays an important role in the control of blood pressure [4].
References:
[1]. Knepper SM, Buckner SA, Brune ME, et al. A-61603, a potent alpha 1-adrenergic receptor agonist, selective for the alpha 1A receptor subtype. J Pharmacol Exp Ther, 1995, 274(1): 97-103.
[2]. Meyer MD, Altenbach RJ, Hancock AA, et al. Synthesis and in vitro characterization of N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8- tetrahydronaphthalen-1-yl]methanesulfonamide and its enantiomers: a novel selective alpha 1A receptor agonist. J Med Chem, 1996, 39(20): 4116-4119.
[3]. Luo DL, Gao J, Fan LL, et al. Receptor subtype involved in alpha 1-adrenergic receptor-mediated Ca2+ signaling in cardiomyocytes. Acta Pharmacol Sin, 2007, 28(7): 968-974.
[4]. Martínez-Salas SG1, Campos-Peralta JM, Pares-Hipolito J, et al. Alpha1A-adrenoceptors predominate in the control of blood pressure in mouse mesenteric vascular bed. Auton Autacoid Pharmacol, 2007, 27(3): 137-142.
- Zafirlukast
Catalog No.:BCC4881
CAS No.:107753-78-6
- Methyl 7beta,15-dihydroxydehydroabietate
Catalog No.:BCN7270
CAS No.:107752-10-3
- Epleremone
Catalog No.:BCC3776
CAS No.:107724-20-9
- MDL 11,939
Catalog No.:BCC6822
CAS No.:107703-78-6
- beta-Lipoic acid
Catalog No.:BCC9198
CAS No.:6992-30-9
- Merucathine
Catalog No.:BCN1782
CAS No.:107673-74-5
- Bulleyaconitine A
Catalog No.:BCN1210
CAS No.:107668-79-1
- Merucathinone
Catalog No.:BCN1783
CAS No.:107638-80-2
- Erycibelline
Catalog No.:BCN1876
CAS No.:107633-95-4
- Dehydroformouregine
Catalog No.:BCN4054
CAS No.:107633-69-2
- PF 998425
Catalog No.:BCC7811
CAS No.:1076225-27-8
- 4-Hydroxycoumarin
Catalog No.:BCN2561
CAS No.:1076-38-6
- Coccinic acid
Catalog No.:BCN5877
CAS No.:107783-45-9
- 3,4-Dihydroxybenzenepropanoic acid
Catalog No.:BCN8500
CAS No.:1078-61-1
- PF 04418948
Catalog No.:BCC6299
CAS No.:1078166-57-0
- Exemestane
Catalog No.:BCC1061
CAS No.:107868-30-4
- Quinovin
Catalog No.:BCN5878
CAS No.:107870-05-3
- Dehydrodiconiferyl alcohol 4-O-beta-D-glucopyranoside
Catalog No.:BCN7707
CAS No.:107870-88-2
- Japonicones D
Catalog No.:BCN3614
CAS No.:1078711-42-8
- Isoeleutherin
Catalog No.:BCN8315
CAS No.:1078723-14-4
- Ganodermanondiol
Catalog No.:BCN5879
CAS No.:107900-76-5
- Sibiricose A5
Catalog No.:BCN2785
CAS No.:107912-97-0
- 9,9-Bis(4-amino-3-methylphenyl)fluorene
Catalog No.:BCC8794
CAS No.:107934-60-1
- Myricananin A
Catalog No.:BCN5880
CAS No.:1079941-35-7
Receptor subtype involved in alpha 1-adrenergic receptor-mediated Ca2+ signaling in cardiomyocytes.[Pubmed:17588332]
Acta Pharmacol Sin. 2007 Jul;28(7):968-74.
AIM: The enhancement of intracellular Ca2+ signaling in response to alpha 1-adrenergic receptor (alpha 1-AR) stimulation is an essential signal transduction event in the regulation of cardiac functions, such as cardiac growth, cardiac contraction, and cardiac adaptation to various situations. The present study was intended to determine the role(s) of the alpha 1-AR subtype(s) in mediating this response. METHODS: We evaluated the effects of subtype-specific agonists and antagonists of the alpha 1- AR on the intracellular Ca2+ signaling of neonatal rat ventricular myocytes using a confocal microscope. RESULTS: After being cultured for 48 h, the myocytes exhibited spontaneous local Ca2+ release, sparks, and global Ca2+ transients. The activation of the alpha 1-AR with phenylephrine, a selective agonist of the alpha 1-AR, dose-dependently increased the frequency of Ca2+ transients with an EC50 value of 2.3 micromol/L. Blocking the alpha 1A-AR subtype with 5-methylurapidil (5-Mu) inhibited the stimulatory effect of phenylephrine with an IC(50) value of 6.7 nmol/L. In contrast, blockade of the alpha 1B-AR and alpha 1D-AR subtypes with chloroethylclonidine and BMY 7378, respectively, did not affect the phenylephrine effect. Similarly, the local Ca2+ spark numbers were also increased by the activation of the alpha 1-AR, and this effect could be abolished selectively by 5-Mu. More importantly, A61603, a novel selective alpha 1A-AR agonist, mimicked the effects of phenylephrine, but with more potency (EC(50) value =6.9 nmol/L) in the potentiation of Ca2+ transients, and blockade of the alpha 1A-AR by 5-Mu caused abolishment of its effects. CONCLUSION: These results indicate that alpha 1-adrenergic stimulation of intracellular Ca2+ activity is mediated selectively by the alpha 1A-AR.
Synthesis and in vitro characterization of N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8- tetrahydronaphthalen-1-yl]methanesulfonamide and its enantiomers: a novel selective alpha 1A receptor agonist.[Pubmed:8831777]
J Med Chem. 1996 Sep 27;39(20):4116-9.
The existence of multiple subtypes of the alpha 1 adrenergic receptor has been demonstrated both pharmacologically and by molecular biological cloning techniques. The development of subtype selective antagonists has been the focus of much research within the pharmaceutical industry, and clinical evidence now exists that alpha-1A selective antagonists will have utility in the treatment of benign prostatic hyperplasia. However, highly subtype selective agonists are not known. Herein we report the synthesis and pharmacological characterization of N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8- tetrahydronaphthalen-1-yl]methanesulfonamide and its enantiomers, a highly potent full agonist with excellent selectivity for the alpha 1A receptor subtype.
A-61603, a potent alpha 1-adrenergic receptor agonist, selective for the alpha 1A receptor subtype.[Pubmed:7616455]
J Pharmacol Exp Ther. 1995 Jul;274(1):97-103.
N-[5-(4,5-dihydro-1H-imidazol-2yl)-2-hydroxy-5,6,7,8-tetrahydro naphthalen-1-yl] methanesulfonamide hydrobromide (A-61603) is a novel and potent alpha-adrenoceptor agonist. In radioligand binding assays, the compound is at least 35-fold more potent at alpha 1A/a receptors than at alpha 1b or alpha 1d sites. In fibroblast cells transfected with alpha 1a receptors, A-61603 more potently stimulates phosphoinositide hydrolysis than norepinephrine, and is antagonized by prazosin. A-61603 is less potent in cells transfected with alpha 1b or alpha 1d receptors. A-61603 is a potent agonist at alpha 1A receptors in rat vas deferens (200- to 300-fold more potent than norepinephrine or phenylephrine, respectively) and in isolated canine prostate strips (130- to 165-fold more potent than norepinephrine or phenylephrine, respectively). In contrast, A-61603 is only 40-fold more potent than phenylephrine at alpha 1B sites in rat spleen and 35-fold less potent at rat aortic, alpha 1D sites. In an in vivo dog model, A-61603 raises intraurethral prostatic tone to a greater extent than mean arterial blood pressure. A-61603 induces a pressor response in conscious rats at doses 50- to 100-fold lower than phenylephrine, and the response is not attenuated by pretreatment with CEC, whereas YM-617 causes a 100-fold shift in the response. These results indicate that A-61603 is a potent adrenergic agonist, selective for alpha 1A/a receptors, and may prove a useful probe for studies of adrenergic function and alpha 1 adrenoceptor regulation of physiological functions.


