6,7,8-TrimethoxycoumarinCAS# 6035-49-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6035-49-0 | SDF | Download SDF |
| PubChem ID | 3083928 | Appearance | Cryst. |
| Formula | C12H12O5 | M.Wt | 236.2 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | 6,7,8-Trimethoxycoumarin; Fraxetin dimethyl ether | ||
| Solubility | DMSO : 125 mg/mL (529.17 mM; Need ultrasonic and warming) | ||
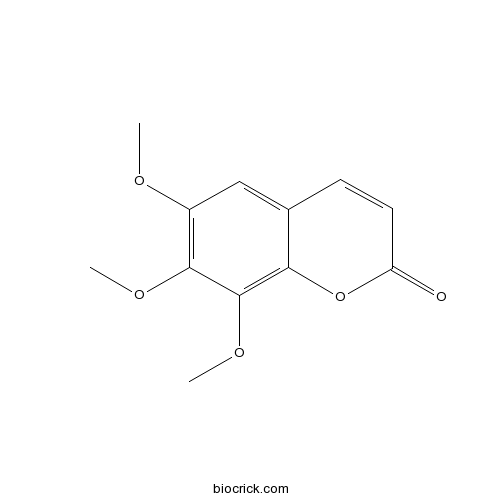
| Chemical Name | 6,7,8-trimethoxychromen-2-one | ||
| SMILES | COC1=C(C(=C2C(=C1)C=CC(=O)O2)OC)OC | ||
| Standard InChIKey | RAYQKHLZHPFYEJ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 6,7,8-Trimethoxycoumarin has antiviral activity, it shows high anti-HRV-2 effect , with IC (50) value of 11.98 muM. It also can improve gastroprotective effects. |
| Targets | P450 (e.g. CYP17) | AChE | Antifection | HRV |
| In vitro | In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens.[Pubmed: 19096995]Planta Med. 2009 Feb;75(3):195-204.The identification of targets whose interaction is likely to result in the successful treatment of a disease is of growing interest for natural product scientists. Antiviral flavonoid from Pterocaulon sphacelatum, an Australian Aboriginal medicine.[Pubmed: 10624889]J Ethnopharmacol. 1999 Dec 15;68(1-3):283-8.The antipicornaviral activity of an ethanolic extract of the green aerial parts of the Australian plant Pterocaulon sphacelatum (Labill.) Benth. & Hook. f. ex F. Muell. has been investigated. This plant has been a favoured traditional medicine, used for the treatment of colds by the Australian Aboriginal people. |
| In vivo | Gastroprotective efficacy and safety evaluation of scoparone derivatives on experimentally induced gastric lesions in rodents.[Pubmed: 25781220]Nutrients. 2015 Mar 13;7(3):1945-64.Among these compounds, 5,6,7-trimethoxycoumarin and 6,7,8-Trimethoxycoumarin were found to have gastroprotective activity greater than the standard drug rebamipide; 6-methoxy-7,8-methylenedioxycoumarin, 6-methoxy-7,8-(1-methoxy)-methylenedioxycoumarin, 6,7-methylenedioxycoumarin, and 6,7-(1-methoxy)-methylenedioxycoumarin were found to be equipotent or less potent that of rebamipide. Pharmacological studies suggest that the presence of a methoxy group at position C-5 or C-8 of the scoparone's phenyl ring significantly improves gastroprotective activity, whereas the presence of a dioxolane ring at C-6, C-7, or C-8 was found to have decreased activity. |

6,7,8-Trimethoxycoumarin Dilution Calculator

6,7,8-Trimethoxycoumarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2337 mL | 21.1685 mL | 42.337 mL | 84.674 mL | 105.8425 mL |
| 5 mM | 0.8467 mL | 4.2337 mL | 8.4674 mL | 16.9348 mL | 21.1685 mL |
| 10 mM | 0.4234 mL | 2.1169 mL | 4.2337 mL | 8.4674 mL | 10.5843 mL |
| 50 mM | 0.0847 mL | 0.4234 mL | 0.8467 mL | 1.6935 mL | 2.1169 mL |
| 100 mM | 0.0423 mL | 0.2117 mL | 0.4234 mL | 0.8467 mL | 1.0584 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dimethylfraxetin is a Carbonic anhydrase inhibitor, with a Ki value of 0.0097 μM.
In Vitro:At CA I there is one stand out compound being Dimethylfraxetin (compound 17), a nanomolar CA I inhibitor. This trimethoxy coumarin is the most potent of any of the NP coumarins across the six CA isozymes of the present study[1].
References:
[1]. Davis RA, et al. Natural product coumarins that inhibit human carbonic anhydrases. Bioorg Med Chem. 2013 Mar 15;21(6):1539-43.
- Leucovorin Calcium
Catalog No.:BCC1198
CAS No.:6035-45-6
- 9-O-Feruloyllariciresinol
Catalog No.:BCN4112
CAS No.:60337-67-9
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
- GSK-3 inhibitor 1
Catalog No.:BCC4126
CAS No.:603272-51-1
- Sulprostone
Catalog No.:BCC7547
CAS No.:60325-46-4
- 4-Methoxyphenyl beta-D-glucopyranoside
Catalog No.:BCN1403
CAS No.:6032-32-2
- NBI 35965 hydrochloride
Catalog No.:BCC7567
CAS No.:603151-83-3
- Geissoschizine methyl ether
Catalog No.:BCN7736
CAS No.:60314-89-8
- Odanacatib (MK-0822)
Catalog No.:BCC1197
CAS No.:603139-19-1
- Sulforhodamine 101
Catalog No.:BCC8019
CAS No.:60311-02-6
- Tamarixetin
Catalog No.:BCN4116
CAS No.:603-61-2
- Chrysosplenetin
Catalog No.:BCN4115
CAS No.:603-56-5
- Doronine
Catalog No.:BCN2106
CAS No.:60367-00-2
- 7,8-Benzoflavone
Catalog No.:BCN6538
CAS No.:604-59-1
- Narcissoside
Catalog No.:BCN1263
CAS No.:604-80-8
- Tombozine
Catalog No.:BCN4117
CAS No.:604-99-9
- Cucurbitacin A
Catalog No.:BCN2468
CAS No.:6040-19-3
- Neopetasitenine
Catalog No.:BCN2114
CAS No.:60409-51-0
- Cauloside F
Catalog No.:BCN3848
CAS No.:60451-47-0
- Cytisine Hydrochloride
Catalog No.:BCN8133
CAS No.:6047-01-4
- Pifithrin-β
Catalog No.:BCC5503
CAS No.:60477-34-1
- Serpentinic acid
Catalog No.:BCN4616
CAS No.:605-14-1
- 5,6-Dihydropyridin-2(1H)-one
Catalog No.:BCN4013
CAS No.:6052-73-9
- Zimelidine dihydrochloride
Catalog No.:BCC7173
CAS No.:60525-15-7
Antiviral flavonoid from Pterocaulon sphacelatum, an Australian Aboriginal medicine.[Pubmed:10624889]
J Ethnopharmacol. 1999 Dec 15;68(1-3):283-8.
The antipicornaviral activity of an ethanolic extract of the green aerial parts of the Australian plant Pterocaulon sphacelatum (Labill.) Benth. & Hook. f. ex F. Muell. has been investigated. This plant has been a favoured traditional medicine, used for the treatment of colds by the Australian Aboriginal people. Antiviral activity-guided fractionation of the extract of P. sphacelatum using an inhibition of poliovirus-induced cytopathic effect assay, has yielded the antiviral flavonoid chrysosplenol C (3,7,3'-trimethoxy-5,6,4'-trihydroxyflavone). This compound is a 4'-hydroxy-3-methoxyflavone, one of a group of compounds known to be potent and specific inhibitors of picornaviral replication. These compounds inhibit the replication of rhinoviruses, the most frequent causative agent of the common cold. The coumarin 6,7,8-Trimethoxycoumarin was also isolated from the ethanolic extract.
In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens.[Pubmed:19096995]
Planta Med. 2009 Feb;75(3):195-204.
The identification of targets whose interaction is likely to result in the successful treatment of a disease is of growing interest for natural product scientists. In the current study we performed an exemplary application of a virtual parallel screening approach to identify potential targets for 16 secondary metabolites isolated and identified from the aerial parts of the medicinal plant RUTA GRAVEOLENS L. Low energy conformers of the isolated constituents were simultaneously screened against a set of 2208 pharmacophore models generated in-house for the IN SILICO prediction of putative biological targets, i. e., target fishing. Based on the predicted ligand-target interactions, we focused on three biological targets, namely acetylcholinesterase (AChE), the human rhinovirus (HRV) coat protein and the cannabinoid receptor type-2 (CB (2)). For a critical evaluation of the applied parallel screening approach, virtual hits and non-hits were assayed on the respective targets. For AChE the highest scoring virtual hit, arborinine, showed the best inhibitory IN VITRO activity on AChE (IC (50) 34.7 muM). Determination of the anti-HRV-2 effect revealed 6,7,8-Trimethoxycoumarin and arborinine to be the most active antiviral constituents with IC (50) values of 11.98 muM and 3.19 muM, respectively. Of these, arborinine was predicted virtually. Of all the molecules subjected to parallel screening, one virtual CB (2) ligand was obtained, i. e., rutamarin. Interestingly, in experimental studies only this compound showed a selective activity to the CB (2) receptor ( Ki of 7.4 muM) by using a radioligand displacement assay. The applied parallel screening paradigm with constituents of R. GRAVEOLENS on three different proteins has shown promise as an IN SILICO tool for rational target fishing and pharmacological profiling of extracts and single chemical entities in natural product research.
Gastroprotective efficacy and safety evaluation of scoparone derivatives on experimentally induced gastric lesions in rodents.[Pubmed:25781220]
Nutrients. 2015 Mar 13;7(3):1945-64.
This study investigated the gastroprotective efficacy of synthesized scoparone derivatives on experimentally induced gastritis and their toxicological safety. Six scoparone derivatives were synthesized and screened for gastroprotective activities against HCl/ethanol- and indomethacin-induced gastric ulcers in rats. Among these compounds, 5,6,7-trimethoxycoumarin and 6,7,8-Trimethoxycoumarin were found to have gastroprotective activity greater than the standard drug rebamipide; 6-methoxy-7,8-methylenedioxycoumarin, 6-methoxy-7,8-(1-methoxy)-methylenedioxycoumarin, 6,7-methylenedioxycoumarin, and 6,7-(1-methoxy)-methylenedioxycoumarin were found to be equipotent or less potent that of rebamipide. Pharmacological studies suggest that the presence of a methoxy group at position C-5 or C-8 of the scoparone's phenyl ring significantly improves gastroprotective activity, whereas the presence of a dioxolane ring at C-6, C-7, or C-8 was found to have decreased activity. In order to assess toxicological safety, two of the potent gastroprotective scoparone derivatives-5,6,7-trimethoxycoumarin and 6,7,8-Trimethoxycoumarin-were examined for their acute toxicity in mice as well as their effect on cytochrome P450 (CYP) enzyme activity. These two compounds showed low acute oral toxicity in adult male and female mice, and caused minimal changes to CYP3A4 and CYP2C9 enzyme activity. These results indicate that compared to other scoparone derivatives, 5,6,7-trimethoxycoumarin and 6,7,8-Trimethoxycoumarin can improve gastroprotective effects, and they have low toxicity and minimal effects on drug-metabolizing enzymes.


