4beta-Hydroxywithanolide ECAS# 54334-04-2 |
Quality Control & MSDS
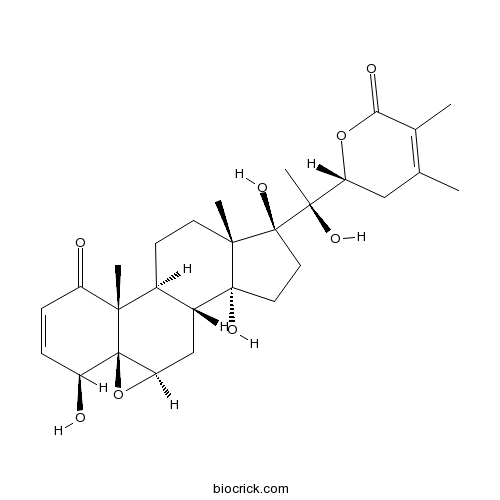
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 54334-04-2 | SDF | Download SDF |
| PubChem ID | 73621 | Appearance | Powder |
| Formula | C28H38O8 | M.Wt | 502.60 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1=C(C(=O)OC(C1)C(C)(C2(CCC3(C2(CCC4C3CC5C6(C4(C(=O)C=CC6O)C)O5)C)O)O)O)C | ||
| Standard InChIKey | UPBUGICUKQIKTJ-KABTZXSUSA-N | ||
| Standard InChI | InChI=1S/C28H38O8/c1-14-12-20(35-22(31)15(14)2)25(5,32)27(34)11-10-26(33)17-13-21-28(36-21)19(30)7-6-18(29)24(28,4)16(17)8-9-23(26,27)3/h6-7,16-17,19-21,30,32-34H,8-13H2,1-5H3/t16-,17+,19-,20+,21+,23-,24-,25-,26+,27-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 4beta-Hydroxywithanolide E(4bHWE) affects alternative splicing by modulating splicing factors and histone modifications, and provides a novel view of the antitumor mechanism of 4bHWE. 2. 4bHWE can inhibit the growth of colon cancer monolayer and spheroid cultures. 3. 4bHWE decreases inflammatory responses by inhibiting the NF-κB signaling in diabetic mouse adipose tissue, it also can improve impaired glucose tolerance, thus, it is a useful natural anti-inflammatory compound to attenuate progression of diabetes and obesity. 4. 4bHWE assert its anti-tumor activity in carcinogenic progression and develop into a dietary chemopreventive agent. |
| Targets | p21 | HSP (e.g. HSP90) | COX | NF-kB | PARP |

4beta-Hydroxywithanolide E Dilution Calculator

4beta-Hydroxywithanolide E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9897 mL | 9.9483 mL | 19.8965 mL | 39.7931 mL | 49.7413 mL |
| 5 mM | 0.3979 mL | 1.9897 mL | 3.9793 mL | 7.9586 mL | 9.9483 mL |
| 10 mM | 0.199 mL | 0.9948 mL | 1.9897 mL | 3.9793 mL | 4.9741 mL |
| 50 mM | 0.0398 mL | 0.199 mL | 0.3979 mL | 0.7959 mL | 0.9948 mL |
| 100 mM | 0.0199 mL | 0.0995 mL | 0.199 mL | 0.3979 mL | 0.4974 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Protopseudohypericin
Catalog No.:BCN2813
CAS No.:54328-09-5
- Amsacrine hydrochloride
Catalog No.:BCC4310
CAS No.:54301-15-4
- Ac-Gly-OH
Catalog No.:BCC2943
CAS No.:543-24-8
- 2',4'-Dihydroxy-3',6'-dimethoxydihydrochalcone
Catalog No.:BCN1421
CAS No.:54299-52-4
- (+)-Fluprostenol
Catalog No.:BCC7947
CAS No.:54276-17-4
- Stachyose trihydrate
Catalog No.:BCN8361
CAS No.:54261-98-2
- 3-Benzoylpyridine
Catalog No.:BCC8623
CAS No.:5424-19-1
- Cimaterol
Catalog No.:BCC6647
CAS No.:54239-37-1
- 1-Monopalmitin
Catalog No.:BCN7749
CAS No.:542-44-9
- Apilimod
Catalog No.:BCC5286
CAS No.:541550-19-0
- Vicriviroc Malate
Catalog No.:BCC1230
CAS No.:541503-81-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Eriodictyol 7,3'-dimethyl ether
Catalog No.:BCN8105
CAS No.:54352-60-2
- Decarine
Catalog No.:BCN5721
CAS No.:54354-62-0
- 4'-Methoxyacetoacetanilide
Catalog No.:BCC8712
CAS No.:5437-98-9
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- 7-Hydroxy-5,8-dimethoxyflavanone
Catalog No.:BCN5722
CAS No.:54377-24-1
- N,N-Bis(2-hydroxyethyl)-p-phenylenediamine sulphate
Catalog No.:BCN8366
CAS No.:54381-16-7
- Lirinidine
Catalog No.:BCN8274
CAS No.:54383-28-7
- Palmitoylethanolamide
Catalog No.:BCC6828
CAS No.:544-31-0
- Myristic acid
Catalog No.:BCN8390
CAS No.:544-63-8
- Norcantharidin
Catalog No.:BCN1281
CAS No.:5442-12-6
- Capadenoson
Catalog No.:BCC1450
CAS No.:544417-40-5
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
Non-homologous end joining pathway is the major route of protection against 4beta-hydroxywithanolide E-induced DNA damage in MCF-7 cells.[Pubmed:24373828]
Food Chem Toxicol. 2014 Mar;65:205-12.
4beta-Hydroxywithanolide E is a bioactive withanolide extracted from Physalis peruviana. 4beta-Hydroxywithanolide E caused reactive oxygen species production and cell apoptosis in human breast cancer MCF-7 cells. We further found that 4beta-Hydroxywithanolide E induced DNA damage and regulated the DNA damage signaling in MCF-7 cells. The DNA damage sensors and repair proteins act promptly to remove DNA lesions by 4beta-Hydroxywithanolide E. The ataxia-telangiectasia mutated protein (ATM)-dependent DNA damage signaling pathway is involved in 4beta-Hydroxywithanolide E-induced apoptosis of MCF-7 cells. Non-homologous end joining pathway, but not homologous recombination, is the major route of protection of MCF-7 cells against 4beta-Hydroxywithanolide E-induced DNA damage. 4beta-Hydroxywithanolide E had no significant impact on the base excision repair pathway. In this study, we examined the 4beta-Hydroxywithanolide E-induced DNA damage as a research tool in project investigating the DNA repair signaling in breast cancer cells. We also suggest that 4beta-Hydroxywithanolide E assert its anti-tumor activity in carcinogenic progression and develop into a dietary chemopreventive agent.
4beta-Hydroxywithanolide E Modulates Alternative Splicing of Apoptotic Genes in Human Hepatocellular Carcinoma Huh-7 Cells.[Pubmed:28779122]
Sci Rep. 2017 Aug 4;7(1):7290.
Alternative splicing is a mechanism for increasing protein diversity from a limited number of genes. Studies have demonstrated that aberrant regulation in the alternative splicing of apoptotic gene transcripts may contribute to the development of cancer. In this study, we isolated 4beta-Hydroxywithanolide E (4bHWE) from the traditional herb Physalis peruviana and investigated its biological effect in cancer cells. The results demonstrated that 4bHWE modulates the alternative splicing of various apoptotic genes, including HIPK3, SMAC/DIABLO, and SURVIVIN. We also discovered that the levels of SRSF1 phospho-isoform were decreased and the levels of H3K36me3 were increased in 4bHWE treatment. Knockdown experiments revealed that the splicing site selection of SMAC/DIABLO could be mediated by changes in the level of H3K36me3 in 4bHWE-treated cells. Furthermore, we extended our study to apoptosis-associated molecules, and detected increased levels of poly ADP-ribose polymerase cleavage and the active form of CASPASE-3 in 4bHWE-induced apoptosis. In vivo experiments indicated that the treatment of tumor-bearing mice with 4bHWE resulted in a marked decrease in tumor size. This study is the first to demonstrate that 4bHWE affects alternative splicing by modulating splicing factors and histone modifications, and provides a novel view of the antitumor mechanism of 4bHWE.
Induction of cell cycle arrest and apoptosis with downregulation of Hsp90 client proteins and histone modification by 4beta-hydroxywithanolide E isolated from Physalis peruviana.[Pubmed:27006100]
Mol Nutr Food Res. 2016 Jun;60(6):1482-500.
SCOPE: Physalis peruviana (Solanaceae) is used for culinary and medicinal purposes. We currently report withanolides, isolated from P. peruviana, inhibit the growth of colon cancer monolayer and spheroid cultures. A detailed mechanistic evaluation was performed with 4beta-Hydroxywithanolide E (4HWE). METHODS AND RESULTS: Treatment of HT-29 cells with low concentrations of 4HWE inhibited growth while enhancing levels of p21(Waf1/Cip1) and reducing levels of several cell cycle-related proteins. Apoptosis was induced at higher concentrations. In addition, 4HWE treatment downregulated the levels of Hsp90 client proteins. Nuclear sirtuin 1 (SIRT1) was increased and histone H3 acetylated at lysine 9 was decreased. An additional consequence of SIRT1 elevation in the nucleus may be inhibition of c-Jun activity. The expression of 21 genes was altered, including downregulation of PTGS2, and this correlated with reduced protein levels of cyclooxygenase-2 (COX-2). Overall, efficacious induction of G0/G1 cell cycle arrest at low concentrations, and induction of apoptosis at higher concentrations are interesting 4HWE-mediated phenomena that are accompanied by a complex array of molecular events. CONCLUSION: Considering the worldwide prevalence of colon cancer, and the unique mode of action mediated by 4HWE, it is reasonable to investigate additional mechanistic details and the potential utility of this compound.
4beta-Hydroxywithanolide E isolated from Physalis pruinosa calyx decreases inflammatory responses by inhibiting the NF-kappaB signaling in diabetic mouse adipose tissue.[Pubmed:24566854]
Int J Obes (Lond). 2014 Nov;38(11):1432-9.
BACKGROUND: Chronic inflammation in adipose tissue together with obesity induces insulin resistance. Inhibitors of chronic inflammation in adipose tissue can be a potent candidate for the treatment of diabetes; however, only a few compounds have been discovered so far. The objective of this study was to find a novel inhibitor that can suppress the inflammatory response in adipose tissue and to elucidate the intracellular signaling mechanisms of the compound. METHODS: To find the active compounds, we established an assay system to evaluate the inhibition of induced MCP-1 production in adipocyte/macrophage coculture in a plant extract library. The active compound was isolated by performing high-performance liquid chromatography (HPLC) and was determined as 4beta-Hydroxywithanolide E (4betaHWE) by nuclear magnetic resonance (NMR) and mass spectroscopy (MS) spectral analyses. The effect of 4betaHWE on inflammation in adipose tissue was assessed with adipocyte culture and db/db mice. RESULTS: During the screening process, Physalis pruinosa calyx extract was found to inhibit production of MCP-1 in coculture strongly. 4betaHWE belongs to the withanolide family of compounds, and it has the strongest MCP-1 production inhibitory effect and lowest toxicity than any other withanolides in coculture. Its anti-inflammatory effect was partially dependent on the attenuation of NF-kappaB signaling in adipocyte. Moreover, in vivo experiments showed that the oral administration of 4betaHWE to db/db mice resulted in the inhibition of macrophage invasion and cytokine expression in adipose tissue after 2 weeks of treatment; improved the plasma adiponectin, non-esterified fatty acids and MCP-1 concentrations; and increased glucose tolerance after 3 to 4 weeks of treatment. CONCLUSIONS: These results suggest that 4betaHWE has anti-inflammatory effect via inhibition of NF-kappaB activation in adipocyte. Moreover, the attenuation of inflammation in adipocyte has an effect on the inhibition of macrophage accumulation in obese adipose tissue. Consequently, 4betaHWE improves impaired glucose tolerance. Thus, 4betaHWE is a useful natural anti-inflammatory compound to attenuate progression of diabetes and obesity.


