3-AQC5-HT3 antagonist CAS# 201216-42-4 |
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 201216-42-4 | SDF | Download SDF |
| PubChem ID | 87671288 | Appearance | Powder |
| Formula | C20H21N5O4 | M.Wt | 395.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
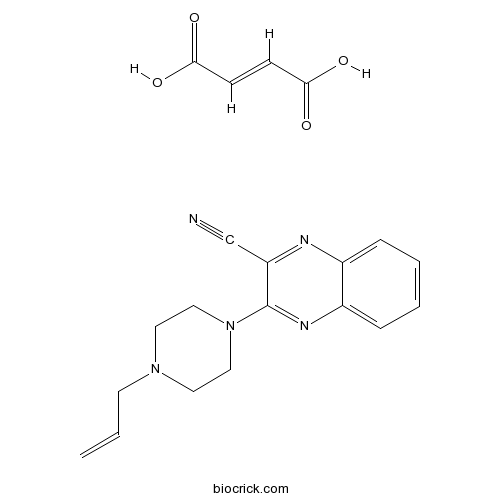
| Chemical Name | (E)-but-2-enedioic acid;3-(4-prop-2-enylpiperazin-1-yl)quinoxaline-2-carbonitrile | ||
| SMILES | C=CCN1CCN(CC1)C2=NC3=CC=CC=C3N=C2C#N.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | UHLVYEOCPBNJNA-WLHGVMLRSA-N | ||
| Standard InChI | InChI=1S/C16H17N5.C4H4O4/c1-2-7-20-8-10-21(11-9-20)16-15(12-17)18-13-5-3-4-6-14(13)19-16;5-3(6)1-2-4(7)8/h2-6H,1,7-11H2;1-2H,(H,5,6)(H,7,8)/b;2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A competitive 5-HT3 antagonist, almost 100 times more potent than tropisetron, but with widely differing activity in various tissues. |

3-AQC Dilution Calculator

3-AQC Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.529 mL | 12.6448 mL | 25.2896 mL | 50.5791 mL | 63.2239 mL |
| 5 mM | 0.5058 mL | 2.529 mL | 5.0579 mL | 10.1158 mL | 12.6448 mL |
| 10 mM | 0.2529 mL | 1.2645 mL | 2.529 mL | 5.0579 mL | 6.3224 mL |
| 50 mM | 0.0506 mL | 0.2529 mL | 0.5058 mL | 1.0116 mL | 1.2645 mL |
| 100 mM | 0.0253 mL | 0.1264 mL | 0.2529 mL | 0.5058 mL | 0.6322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-PPBP maleate
Catalog No.:BCC6723
CAS No.:201216-39-9
- 2-Amino-2'-chloro-5-nitro benzophenone
Catalog No.:BCC8521
CAS No.:2011-66-7
- cis-Methylkhellactone
Catalog No.:BCN7690
CAS No.:20107-13-5
- Ravenine
Catalog No.:BCN6666
CAS No.:20105-22-0
- SB-269970
Catalog No.:BCC1927
CAS No.:201038-74-6
- Fmoc-Lys(Me)3-OH Chloride
Catalog No.:BCC3267
CAS No.:201004-29-7
- Ac-RYYRIK-NH2
Catalog No.:BCC5736
CAS No.:200959-48-4
- Ac-RYYRWK-NH2
Catalog No.:BCC5755
CAS No.:200959-47-3
- SB 243213 dihydrochloride
Catalog No.:BCC6035
CAS No.:200940-23-4
- m-3M3FBS
Catalog No.:BCC7209
CAS No.:200933-14-8
- (D)-(+)-Neopterin
Catalog No.:BCC7960
CAS No.:2009-64-5
- Xanthotoxol
Catalog No.:BCN4881
CAS No.:2009-24-7
- Diosmetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN5328
CAS No.:20126-59-4
- H-Thr-OBzl.oxalate
Catalog No.:BCC3103
CAS No.:201274-07-9
- 1-(3,4-Dimethoxyphenyl)propane-1,2-diol
Catalog No.:BCN1507
CAS No.:20133-19-1
- Fmoc-D-Tyr(Me)-OH
Catalog No.:BCC3269
CAS No.:201335-88-8
- Tenofovir disoproxil
Catalog No.:BCN2178
CAS No.:201341-05-1
- Rutundic acid
Catalog No.:BCN5370
CAS No.:20137-37-5
- Talarozole
Catalog No.:BCC1980
CAS No.:201410-53-9
- PKI 14-22 amide, myristoylated
Catalog No.:BCC8087
CAS No.:201422-03-9
- Boc-Dap(Boc)-OH.DCHA
Catalog No.:BCC2664
CAS No.:201472-68-6
- Fmoc-Asn-ol
Catalog No.:BCC2586
CAS No.:201484-12-0
- Dilazep dihydrochloride
Catalog No.:BCC6660
CAS No.:20153-98-4
- Deferasirox
Catalog No.:BCC3924
CAS No.:201530-41-8
The pre-nervous serotonergic system of developing sea urchin embryos and larvae: pharmacologic and immunocytochemical evidence.[Pubmed:16187217]
Neurochem Res. 2005 Jun-Jul;30(6-7):825-37.
Forty serotonin-related neurochemicals were tested on embryos and larvae of Lytechinus variegatus and other sea urchin species. Some of these substances (agonists of 5-HT1 receptors, antagonists of 5-HT2, 5-HT3 or 5-HT4 receptors, and inhibitors of the serotonin transporter, SERT) perturbed post-blastulation development, eliciting changes in embryonic/larval phenotypes typical for each class of receptor ligand. These developmental malformations were prevented completely or partially by serotonin (5-HT) or 5-HT analogs (5-HTQ, AA-5-HT), providing evidence for the putative localization of cellular targets. Immunoreactive 5-HT, 5-HT receptors and SERT were found in pre-nervous embryos and larvae of both L. variegatus and Strongylocentrotus droebachiensis. During gastrulation, these components of the serotonergic system were localized to the archenteron (primary gut), mesenchyme-like cells, and often the apical ectoderm. These results provide evidence that pre-nervous 5-HT may regulate early events of sea urchin embryogenesis, mediated by 5-HT receptors or the 5-HT transporter.
5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes.[Pubmed:15802305]
Int Immunol. 2005 May;17(5):599-606.
The neurotransmitter 5-hydroxytryptamine (5-HT), commonly known as serotonin, is released at peripheral sites from activated enterochromaffin cells, mast cells and platelets. In this study we analyzed the biological activity and intracellular signaling of 5-HT in human monocytes. By reverse transcription (RT) and PCR, messenger RNA (mRNA) expression of 5-HT receptor 1E (5-HTR(1E)), 5-HTR(2A), 5-HTR(3), 5-HTR(4) and 5-HTR(7) could be revealed. Functional studies showed that 5-HT modulates the release of IL-1beta, IL-6, IL-8/CXCL8, IL-12p40 and tumor necrosis factor-alpha (TNF-alpha), while it has no effect on the production of IL-18 and IFN-gamma in LPS-stimulated human blood monocytes. Moreover, RT and PCR revealed that 5-HT modulated mRNA levels of IL-6 and IL-8/CXCL8, but did not influence mRNA levels of IL-1beta and TNF-alpha. Pharmacological studies with isotype-selective receptor agonists allowed us to show that 5-HTR(3) subtype up-regulates the LPS-induced production of IL-1beta, IL-6 and IL-8/CXCL8, while it was not involved in TNF-alpha and IL-12p40 secretion. Furthermore, activation of the G(s)-coupled 5-HTR(4) and 5-HTR(7) subtypes increased intracellular cyclic AMP (cAMP) and secretion of IL-1beta, IL-6, IL-12p40 and IL-8/CXCL8, while, on the contrary, it inhibited LPS-induced TNF-alpha release. Interestingly, 5-HTR(1) and 5-HTR(2) agonists did not modulate the LPS-induced cytokine production in human monocytes. Our results point to a new role for 5-HT in inflammation by modulating cytokine production in monocytes via activation of 5-HTR(3), 5-HTR(4) and 5-HTR(7) subtypes.
Novel antagonists of 5-HT3 receptors. Synthesis and biological evaluation of piperazinylquinoxaline derivatives.[Pubmed:8410988]
J Med Chem. 1993 Sep 17;36(19):2745-50.
A series of piperazinylquinoxalines has been synthesized and studied as 5-HT3 receptor antagonists in different preparations. Antagonism to 5-HT in the longitudinal muscle of the guinea pig ileum was particularly prominent in cyanoquinoxaline derivatives with an alkyl substitutuent on the piperazine moiety. The pA2 of some selected compounds against the 5-HT3 agonist 2-methyl-5HT in the guinea pig ileum was in the range of tropisetron or ondansetron, and one of them, 7e, was more potent than these reference compounds by approximately 2 or 3 orders of magnitude. However, these compounds were markedly less potent than either tropisetron or ondansetron as displacers of 3H-BRL 43694 binding to rat cortical membranes or as antagonists of the Bezold-Jarisch reflex in rats. Piperazinylcyanoquinoxalines represent a new class of 5-HT3 antagonists with a selective effect on guinea pig peripheral receptors.


