pep2mPeptide inhibitor of GluR2 subunit binding to NSF. Reduces AMPA currents CAS# 243843-42-7 |
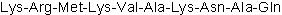
- CGS 21680
Catalog No.:BCC1475
CAS No.:120225-54-9
- CGS 21680 HCl
Catalog No.:BCC4316
CAS No.:124431-80-7
- Valsartan
Catalog No.:BCC5017
CAS No.:137862-53-4
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- Ticlopidine HCl
Catalog No.:BCC4973
CAS No.:53885-35-1
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 243843-42-7 | SDF | Download SDF |
| PubChem ID | 90479806 | Appearance | Powder |
| Formula | C49H92N18O13S | M.Wt | 1173.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GluR2<sub>m</sub>, G10 | ||
| Solubility | Soluble to 2 mg/ml in 20% acetonitrile | ||
| Sequence | KRMKVAKNAQ | ||
| Chemical Name | (2S)-5-amino-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2,6-diaminohexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-4-methylsulfanylbutanoyl]amino]hexanoyl]amino]-3-methylbutanoyl]amino]propanoyl]amino]hexanoyl]amino]-4-oxobutanoyl]amino]propanoyl]amino]-5-oxopentanoic acid | ||
| SMILES | CC(C)C(C(=O)NC(C)C(=O)NC(CCCCN)C(=O)NC(CC(=O)N)C(=O)NC(C)C(=O)NC(CCC(=O)N)C(=O)O)NC(=O)C(CCCCN)NC(=O)C(CCSC)NC(=O)C(CCCN=C(N)N)NC(=O)C(CCCCN)N | ||
| Standard InChIKey | DTYSOEUWYZLCBY-MFTMZZJWSA-N | ||
| Standard InChI | InChI=1S/C49H92N18O13S/c1-26(2)38(67-45(76)31(15-8-11-22-52)63-44(75)33(19-24-81-5)64-42(73)32(16-12-23-58-49(56)57)62-41(72)29(53)13-6-9-20-50)47(78)60-28(4)39(70)61-30(14-7-10-21-51)43(74)66-35(25-37(55)69)46(77)59-27(3)40(71)65-34(48(79)80)17-18-36(54)68/h26-35,38H,6-25,50-53H2,1-5H3,(H2,54,68)(H2,55,69)(H,59,77)(H,60,78)(H,61,70)(H,62,72)(H,63,75)(H,64,73)(H,65,71)(H,66,74)(H,67,76)(H,79,80)(H4,56,57,58)/t27-,28-,29-,30-,31-,32-,33-,34-,35-,38-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peptide inhibitor of the interaction between the C-terminus of the GluR2 (AMPA receptor) subunit and N-ethylmaleimide-sensitive fusion protein (NSF), a protein that regulates AMPA receptor function. Reduces postsynaptic currents in CA1 neurons, AMPA-mediated currents in cultured hippocampal neurons and AMPA receptor surface expression. Control peptide pep4c also available. |

pep2m Dilution Calculator

pep2m Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-(-)-Fucose
Catalog No.:BCN8326
CAS No.:2438-80-4
- Bufexamac
Catalog No.:BCC4427
CAS No.:2438-72-4
- (-)-alpha-Pinene
Catalog No.:BCC8295
CAS No.:2437-95-8
- 3,5-Cycloergosta-6,8(14),22-triene
Catalog No.:BCN5100
CAS No.:24352-51-0
- S-(5'-Adenosyl)-L-methionine chloride
Catalog No.:BCN2229
CAS No.:24346-00-7
- Apamin
Catalog No.:BCC7141
CAS No.:24345-16-2
- 6-Amino-1-methyluracil
Catalog No.:BCC8757
CAS No.:2434-53-9
- Nagilactone C
Catalog No.:BCN4040
CAS No.:24338-53-2
- Tricosanoic acid
Catalog No.:BCN5394
CAS No.:2433-96-7
- 9-Fluorenylmethanol
Catalog No.:BCC2801
CAS No.:24324-17-2
- Cephalotaxine
Catalog No.:BCN2957
CAS No.:24316-19-6
- Scandine
Catalog No.:BCN5099
CAS No.:24314-59-8
- pep4c
Catalog No.:BCC5783
CAS No.:243843-43-8
- Glycoside L-F2
Catalog No.:BCN2158
CAS No.:243857-99-0
- 5-Iodotubercidin
Catalog No.:BCC1312
CAS No.:24386-93-4
- Kynurenic acid sodium salt
Catalog No.:BCC7754
CAS No.:2439-02-3
- Ethyl 4-methoxycinnamate
Catalog No.:BCN5028
CAS No.:24393-56-4
- Cnicin
Catalog No.:BCN8546
CAS No.:24394-09-0
- TCS 401
Catalog No.:BCC2469
CAS No.:243967-42-2
- TAK-242 S enantiomer
Catalog No.:BCC1978
CAS No.:243984-10-3
- TAK-242
Catalog No.:BCC1977
CAS No.:243984-11-4
- beta-D-glucose
Catalog No.:BCN8171
CAS No.:492-61-5
- (+)-Epipinoresinol
Catalog No.:BCN3255
CAS No.:24404-50-0
- Beta-Rotunol
Catalog No.:BCN6628
CAS No.:24405-57-0
Hippocampal Long-Term Depression in the Presence of Calcium-Permeable AMPA Receptors.[Pubmed:30483111]
Front Synaptic Neurosci. 2018 Nov 13;10:41.
The GluA2 subunit of AMPA glutamate receptors (AMPARs) has been shown to be critical for the expression of NMDA receptor (NMDAR)-dependent long-term depression (LTD). However, in young GluA2 knockout (KO) mice, this form of LTD can still be induced in the hippocampus, suggesting that LTD mechanisms may be modified in the presence of GluA2-lacking, Ca(2+) permeable AMPARs. In this study, we examined LTD at the CA1 synapse in GluA2 KO mice by using several well-established inhibitory peptides known to block LTD in wild type (WT) rodents. We showed that while LTD in the KO mice is still blocked by the protein interacting with C kinase 1 (PICK1) peptide pepEVKI, it becomes insensitive to the N-ethylmaleimide-sensitive factor (NSF) peptide pep2m. In addition, the effects of actin and cofilin inhibitory peptides were also altered. These results indicate that in the absence of GluA2, LTD expression mechanisms are different from those in WT animals, suggesting that there are multiple molecular processes enabling LTD expression that are adaptable to physiological and genetic manipulations.
Presynaptic PICK1 facilitates trafficking of AMPA-receptors between active zone and synaptic vesicle pool.[Pubmed:28057533]
Neuroscience. 2017 Mar 6;344:102-112.
Previous studies have indicated that presynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors (AMPARs) contribute to the regulation of neurotransmitter release. In hippocampal synapses, the presynaptic surface expression of several AMPAR subunits, including GluA2, is regulated in a ligand-dependent manner. However, the molecular mechanisms underlying the presynaptic trafficking of AMPARs are still unknown. Here, using bright-field immunocytochemistry, western blots, and quantitative immunogold electron microscopy of the hippocampal CA1 area from intact adult rat brain, we demonstrate the association of AMPA receptors with the presynaptic active zone and with small presynaptic vesicles, in Schaffer collateral synapses in CA1 of the hippocampus. Furthermore, we show that GluA2 and protein interacting with C kinase 1 (PICK1) are colocalized at presynaptic vesicles. Similar to postsynaptic mechanisms, overexpression of either PICK1 or pep2m, which inhibit the N-ethylmaleimide sensitive fusion protein (NSF)-GluA2 interaction, decreases the concentration of GluA2 in the presynaptic active zone membrane. These data suggest that the interacting proteins PICK1 and NSF act as regulators of presynaptic GluA2-containing AMPAR trafficking between the active zone and a vesicle pool that may provide the basis of presynaptic components of synaptic plasticity.
Evidence of calcium-permeable AMPA receptors in dendritic spines of CA1 pyramidal neurons.[Pubmed:24760782]
J Neurophysiol. 2014 Jul 15;112(2):263-75.
GluA2-lacking, calcium-permeable alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors (AMPARs) have unique properties, but their presence at excitatory synapses in pyramidal cells is controversial. We have tested certain predictions of the model that such receptors are present in CA1 cells and show here that the polyamine spermine, but not philanthotoxin, causes use-dependent inhibition of synaptically evoked excitatory responses in stratum radiatum, but not s. oriens, in cultured and acute hippocampal slices. Stimulation of single dendritic spines by photolytic release of caged glutamate induced an N-methyl-d-aspartate receptor-independent, use- and spermine-sensitive calcium influx only at apical spines in cultured slices. Bath application of glutamate also triggered a spermine-sensitive influx of cobalt into CA1 cell dendrites in s. radiatum. Responses of single apical, but not basal, spines to photostimulation displayed prominent paired-pulse facilitation (PPF) consistent with use-dependent relief of cytoplasmic polyamine block. Responses at apical dendrites were diminished, and PPF was increased, by spermine. Intracellular application of pep2m, which inhibits recycling of GluA2-containing AMPARs, reduced apical spine responses and increased PPF. We conclude that some calcium-permeable, polyamine-sensitive AMPARs, perhaps lacking GluA2 subunits, are present at synapses on apical dendrites of CA1 pyramidal cells, which may allow distinct forms of synaptic plasticity and computation at different sets of excitatory inputs.
The maintenance of long-term memory in the hippocampus depends on the interaction between N-ethylmaleimide-sensitive factor and GluA2.[Pubmed:24753224]
Hippocampus. 2014 Sep;24(9):1112-9.
The maintenance of established memories has recently been shown to involve the stabilization of GluA2-containing AMPA receptors (GluA2/AMPARs) at postsynaptic membranes. Previous studies have suggested that N-ethylmaleimide-sensitive factor (NSF) regulates the stabilization of AMPARs at the synaptic membrane. We therefore disrupted the interaction between GluA2 and NSF in the dorsal hippocampus and examined its effect on the maintenance of object location and contextual fear memory. We used two interference peptides, pep2m and pepR845A, that have been shown to block the binding of NSF to GluA2 and reduce GluA2 synaptic content. Either peptide disrupted consolidated memory, and these effects persisted for at least 5 or 28 days after peptide administration. Following peptide administration and long-term memory disruption, rats were able to acquire new memories. Memory acquisition or consolidation was not impaired when pepR845A was given immediately before the training sessions. Blocking GluA2 endocytosis with the peptide GluA23Y prevented the memory impairment effect of pepR845A. Taken together, our results indicate that the persistence of long-term memory depends on the maintenance of a steady-state level of synaptic GluA2/AMPARs, which requires the interaction of NSF with GluA2.
Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action.[Pubmed:19890264]
Neuropsychopharmacology. 2010 Feb;35(3):674-85.
Stress and glucocorticoids (GCs) can facilitate memory formation. However, the molecular mechanisms mediating their effects are largely unknown. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor (AMPAR) trafficking has been implicated in the changes in synaptic strength at central glutamatergic synapses associated with memory formation. In cell cultures, corticosterone has been shown to condition the synaptic trafficking of the AMPAR GluA2 subunit. In this study, we investigated the involvement of GluA2 trafficking in the facilitation of learning by stress. Using the water maze spatial task involving different stress levels, mice trained under more stressful conditions (water at 22 degrees C) showed better learning and memory, and higher post-training corticosterone levels, than mice trained under lower stress (water at 30 degrees C). Strikingly, this facilitated learning by stress was accompanied by enhanced synaptic expression of GluA2 AMPARs that was not observed in mice trained under less stressful conditions. Interfering with GC actions by injecting the GC synthesis inhibitor, metyrapone, blocked both the memory facilitation and the enhanced GluA2 trafficking induced by stressful learning. Intracerebroventricular infusion of the peptide, pep2m, that blocks GluA2 synaptic trafficking by interfering with the interaction between N-ethylmaleimide-sensitive factor and GluA2, impaired immediate performance at learning as well as long-term memory retrieval, supporting a causal role for GluA2 trafficking in stress-induced facilitation of spatial learning and memory. Evidence for the involvement of the neural cell adhesion molecule N-cadherin in interaction with GluA2 is also provided. These findings underscore a new mechanism whereby stress can improve memory function.
Trafficking of presynaptic AMPA receptors mediating neurotransmitter release: neuronal selectivity and relationships with sensitivity to cyclothiazide.[Pubmed:16242162]
Neuropharmacology. 2006 Mar;50(3):286-96.
Postsynaptic glutamate AMPA receptors (AMPARs) can recycle between plasma membrane and intracellular pools. In contrast, trafficking of presynaptic AMPARs has not been investigated. AMPAR surface expression involves interactions between the GluR2 carboxy tail and various proteins including glutamate receptor-interacting protein (GRIP), AMPA receptor-binding protein (ABP), protein interacting with C kinase 1 (PICK1), N-ethyl-maleimide-sensitive fusion protein (NSF). Here, peptides known to selectively block the above interactions were entrapped into synaptosomes to study the effects on the AMPA-evoked release of [3H]noradrenaline ([3H]NA) and [3H]acetylcholine ([3H]ACh) from rat hippocampal and cortical synaptosomes, respectively. Internalization of pep2-SVKI to prevent GluR2-GRIP/ABP/PICK1 interactions potentiated the AMPA-evoked release of [3H]NA but left unmodified that of [3H]ACh. Similar potentiation was caused by pep2-AVKI, the blocker of GluR2-PICK1 interaction. Conversely, a decrease in the AMPA-evoked release of [3H]NA, but not of [3H]ACh, was caused by pep2m, a selective blocker of the GluR2-NSF interaction. In the presence of pep2-SVKI the presynaptic AMPARs on noradrenergic terminals lost sensitivity to cyclothiazide. AMPARs releasing [3H]ACh, but not those releasing [3H]NA, were sensitive to spermine, suggesting that they are GluR2-lacking AMPARs. To conclude: (i) release-regulating presynaptic AMPARs constitutively cycle in isolated nerve terminals; (ii) the process exhibits neuronal selectivity; (iii) AMPAR trafficking and desensitization may be interrelated.
Two Loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses.[Pubmed:14999062]
J Neurosci. 2004 Mar 3;24(9):2112-21.
Two distinct forms of long-term depression (LTD) exist at mossy fiber synapses between dentate gyrus granule cells and hippocampal CA3 stratum lucidum interneurons. Although induction of each form of LTD requires an elevation of postsynaptic intracellular Ca2+, at Ca2+-impermeable AMPA receptor (CI-AMPAR) synapses, induction is NMDA receptor (NMDAR) dependent, whereas LTD at Ca2+-permeable AMPA receptor (CP-AMPAR) synapses is NMDAR independent. However, the expression locus of either form of LTD is not known. Using a number of criteria, including the coefficient of variation, paired-pulse ratio, AMPA-NMDA receptor activity, and the low-affinity AMPAR antagonist gamma-D-glutamyl-glycine, we demonstrate that LTD expression at CP-AMPAR synapses is presynaptic and results from reduced transmitter release, whereas LTD expression at CI-AMPAR synapses is postsynaptic. The N-ethylmaleimide-sensitive fusion protein-AP2-clathrin adaptor protein 2 inhibitory peptide pep2m occluded LTD expression at CI-AMPAR synapses but not at CP-AMPAR synapses, confirming that CI-AMPAR LTD involves postsynaptic AMPAR trafficking. Thus, mossy fiber innervation of CA3 stratum lucidum interneurons occurs via two parallel systems targeted to either Ca2+-permeable or Ca2+-impermeable AMPA receptors, each with a distinct expression locus for long-term synaptic plasticity.
Disruption of the GluR2-NSF interaction protects primary hippocampal neurons from ischemic stress.[Pubmed:11312602]
Mol Cell Neurosci. 2001 Apr;17(4):662-70.
A specific interaction between the AMPA receptor subunits GluR2 and GluR3 and the fusion protein NSF has recently been identified. Disruption of this interaction by adenoviral-mediated expression of a peptide (pep2m) corresponding to the NSF-binding region of GluR2 results in a dramatic reduction in surface expression of AMPA receptors in primary hippocampal neurons. Here we report that expression of pep2m from a recently developed neuronal-specific adenoviral system gave significant neuroprotection to primary CA1-CA3 hippocampal neurons following stimulation with kainate (KA) and this was accompanied by a reduction in Ca(2+) influx. Protection was also observed following glucose deprivation and exposure to ischemic buffer in the absence of any NMDA receptor antagonists. These results provide strong evidence that AMPA receptors play a direct role in mediating postischemic neurotoxicity.
Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction.[Pubmed:10571232]
Neuron. 1999 Oct;24(2):389-99.
We investigated whether the interaction between the N-ethyl-maleimide-sensitive fusion protein (NSF) and the AMPA receptor (AMPAR) subunit GluR2 is involved in synaptic plasticity in the CA1 region of the hippocampus. Blockade of the NSF-GluR2 interaction by a specific peptide (pep2m) introduced into neurons prevented homosynaptic, de novo long-term depression (LTD). Moreover, saturation of LTD prevented the pep2m-induced reduction in AMPAR-mediated excitatory postsynaptic currents (EPSCs). Minimal stimulation experiments indicated that both pep2m action and LTD were due to changes in quantal size and quantal content but were not associated with changes in AMPAR single-channel conductance or EPSC kinetics. These results suggest that there is a pool of AMPARs dependent on the NSF-GluR2 interaction and that LTD expression involves the removal of these receptors from synapses.
Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism.[Pubmed:10399941]
Neuron. 1999 Jun;23(2):365-76.
Here, we show that disruption of N-ethylmaleimide-sensitive fusion protein- (NSF-) GluR2 interaction by infusion into cultured hippocampal neurons of a blocking peptide (pep2m) caused a rapid decrease in the frequency but no change in the amplitude of AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs). N-methyl-D-aspartate (NMDA) receptor-mediated mEPSCs were not changed. Viral expression of pep2m reduced the surface expression of alpha-amino-3-hydroxy-5-methyl-isoxazolepropionate (AMPA) receptors, whereas NMDA receptor surface expression in the same living cells was unchanged. In permeabilized neurons, the total amount of GluR2 immunoreactivity was unchanged, and a punctate distribution of GluR2 was observed throughout the dendritic tree. These data suggest that the NSF-GluR2 interaction is required for the surface expression of GluR2-containing AMPA receptors and that disruption of the interaction leads to the functional elimination of AMPA receptors at synapses.
Functional roles of protein interactions with AMPA and kainate receptors.[Pubmed:12941441]
Neurosci Res. 2003 Sep;47(1):3-15.
The glutamate receptor subtypes AMPA and kainate are involved in synaptic transmission and synaptic plasticity in the CNS. Recently there has been considerable interest in understanding the molecular regulation of these receptors by proteins that directly bind to AMPA and kainate receptor subunits. Amongst the first interaction partners to be discovered were NSF, ABP, GRIP and PICK1, which bind the AMPA receptor subunit GLUA2. We have studied the functional roles of the interactions of these proteins in regulating AMPA receptor-mediated synaptic transmission and synaptic plasticity in the hippocampus. We have also started to investigate the functions of PICK1 and GRIP on kainate receptor-mediated synaptic transmission in this region. In this article we reflect upon this work, which has led to some new ideas about how AMPA and kainate receptors are regulated at synapses.
Role of AMPA receptor cycling in synaptic transmission and plasticity.[Pubmed:10595516]
Neuron. 1999 Nov;24(3):649-58.
Compounds known to disrupt exocytosis or endocytosis were introduced into CA1 pyramidal cells while monitoring excitatory postsynaptic currents (EPSCs). Disrupting exocytosis or the interaction of GluR2 with NSF caused a gradual reduction in the AMPAR EPSC, while inhibition of endocytosis caused a gradual increase in the AMPAR EPSC. These manipulations had no effect on the NMDAR EPSC but prevented the subsequent induction of LTD. These results suggest that AMPARs, but not NMDARs, cycle into and out of the synaptic membrane at a rapid rate and that certain forms of synaptic plasticity may utilize this dynamic process.
NSF binding to GluR2 regulates synaptic transmission.[Pubmed:9697854]
Neuron. 1998 Jul;21(1):87-97.
Here, we show that N-ethylmaleimide-sensitive fusion protein (NSF) interacts directly and selectively with the intracellular C-terminal domain of the GluR2 subunit of AMPA receptors. The interaction requires all three domains of NSF but occurs between residues Lys-844 and Gln-853 of rat GluR2, with Asn-851 playing a critical role. Loading of decapeptides corresponding to the NSF-binding domain of GluR2 into rat hippocampal CA1 pyramidal neurons results in a marked, progressive decrement of AMPA receptor-mediated synaptic transmission. This reduction in synaptic transmission was also observed when an anti-NSF monoclonal antibody (mAb) was loaded into CA1 neurons. These results demonstrate a previously unsuspected direct interaction in the postsynaptic neuron between two major proteins involved in synaptic transmission and suggest a rapid NSF-dependent modulation of AMPA receptor function.


