| Catalog No. | BCN2308 | 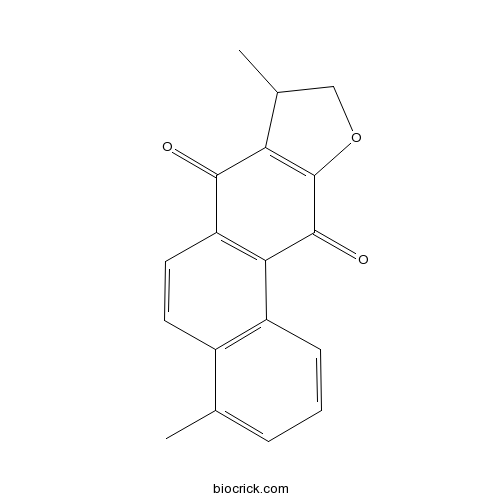 |
|
| CAS RN | 20958-18-3 | ||
| Molecular Weight | 278.30 | ||
| Molecular Formula | C18H14O3 | ||
| Database | [PubChem]:382158293 [ChEBI]: [PCIDB]: |
||
InChI=1S/C18H14O3/c1-9-4-3-5-12-11(9)6-7-13-15(12)17(20)18-14(16(13)19)10(2)8-21-18/h3-7,10H,8H2,1-2H3
Dihydrotanshinone I and cryptotanshinone, constituents of a medicinal plant, Salvia miltiorrhiza Bunge, have antibacterial activity against a broad range of Gram positive bacteria, they have non-selectively inhibition against DNA, RNA, and protein syntheses in B. subtilis, suggest that superoxide radicals are important in the antibacterial actions of the agents.[1]
Dihydrotanshinone I induces topoisomerase I-mediated DNA cleavage as strongly as camptothecin; and inhibits the catalytic activity of topoisomerase I by the formation of a cleavable complex and at least in part through the intercalation into DNA.[2]
Dihydrotanshinone I (DI) has cytotoxicity to a variety of tumor cells, DI (with an IC 50 value of approximately 1.28 ug/ml) could inhibit angio-genesis through suppressing endothelial cell proliferation, migration, invasion and tube formation, indicating that DI has a potential to be developed as a novel anti-angiogenic agent.[3]
Dihydrotanshinone I as an inhibitor of NF-κB activation through our research on Salvia miltiorrhiza Bunge, it significantly inhibits the expression of NF-κB reporter gene induced by TNF-α in a dose-dependent manner, also inhibits TNF-α induced phosphorylation and degradation of IκBα, phosphorylation and nuclear translocation of p65; it suppresses the growth of HeLa cells in a xenograft tumor model, which could be correlated with its modulation of TNF-α production, taken together, dihydrotanshinone I could be a valuable candidate for the intervention of NF-κB-dependent pathological conditions such as inflammation and cancer.[4]
English website: Dihydroisotanshinone I
Japanese website: Dihydroisotanshinone I
Chinese website: Dihydroisotanshinone I
[1] Lee D S, Lee S H, Noh J G, et al. Biosci Biotechn Bioch, 2014, 63(12):2236-9.
[2] Lee D S, Lee S H, Kwon G S, et al. Biosci Biotechn Bioch, 1999, 63(8):1370-3.
[3 ]Weipeng, Bian, Chen, et al. Acta Bioch Et Bioph Sin, 2008, 40(1):1–6.
[4] Wang F, Ma J, Wang KS, et al. Int Immunopharmacol, 2015, 28(1):764-72.
[5] Zhu D, Tan S. China Pharmacist, 2008, 11(03):301-3.


