Rumex nepalensis
Rumex nepalensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Rumex nepalensis
- Cat.No. Product Name CAS Number COA
-
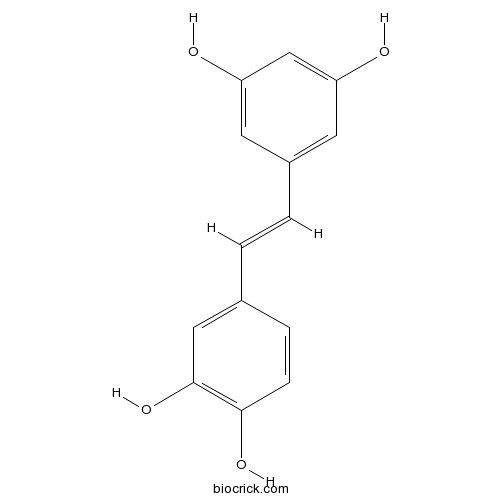
BCN5824
Piceatannol10083-24-6
Instructions
-
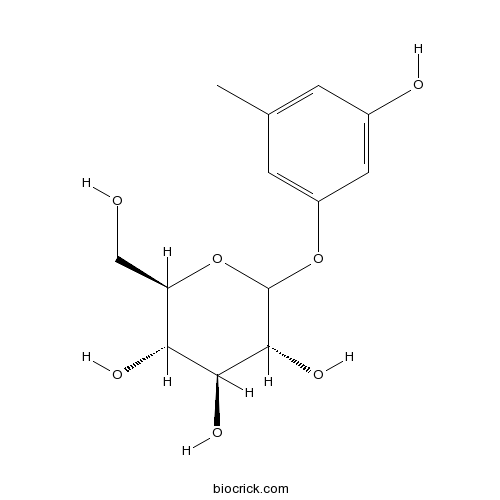
BCN4916
Sakakin21082-33-7
Instructions
-
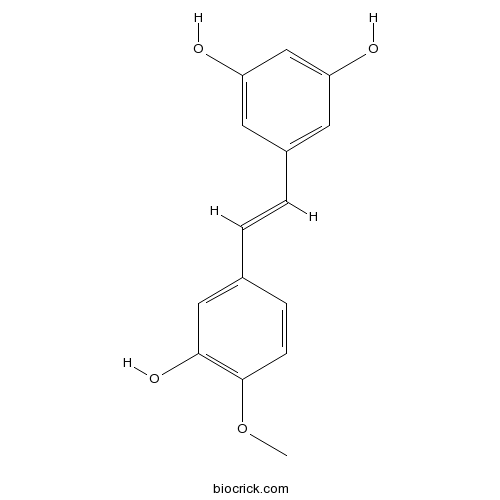
BCN3515
Rhapontigenin500-65-2
Instructions
-
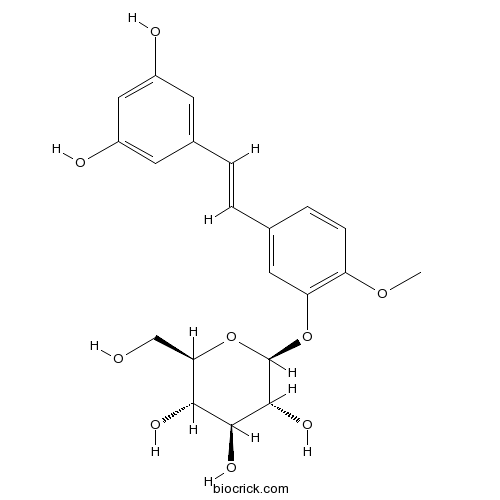
BCN2873
Rhapontigenin 3'-O-glucoside94356-22-6
Instructions
[A new napthalenone from roots of Rumex nepalensis].[Pubmed: 29171233]
A new napthalenone, rumexone A (1), was isolated from the roots of Rumex nepalensis. The structure of 1 was elucidated by extensive spectroscopic analyses, including 1D and 2D NMR spectra and MS data. Its cytotoxic effect was evaluated using four clinically relevant human cancer cell lines, gastric carcinoma SGC7901, breast carcinoma MDA-MB-231, lung carcinoma A549, and hepatocellular carcinoma HepG2.
Himalayan dock (Rumex nepalensis): the flip side of obnoxious weed.[Pubmed: 26543591]
Himalayan dock (Rumex nepalensis) was evaluated for forage value and antinutrients under three, five and seven weeks cutting intervals in the temperate environment. Dry matter (DM) content was measured for each cutting interval. Forage quality parameters such as Crude Protein (CP), Acid Detergent fiber (ADF), Neutral Detergent Fiber (NDF), Calcium (Ca) and Phosphorus (P) were analyzed. Plants with seven weeks cutting interval gave higher DM yield. CP and P content were significantly higher for three weeks cutting intervals. Average CP contents were 31.38 %, 30.73 % and 27.32 % and average P content 0.58 %, 0.52 % and 0.51 % for three, five and seven weeks cutting intervals, respectively. Ca content did not differ significantly between cutting intervals. The average Ca content were 0.91 %, 0.90 % and 90 %, for three, five and seven weeks cutting intervals, respectively. Tannin and mimosine contents were not significantly different between cutting intervals. Average tannin contents were 1.32 %, 1.27 % and 1.26 % and mimosine 0.38 %, 0.30 % and 0.28 % for three, five and seven weeks cutting intervals, respectively. The study concluded that R. nepalensis could be a potential source of protein for livestock. The study also suggests seven weeks harvesting interval to provide plants with high dry matter yield, high forage quality and very low levels of anti-nutrients.
Synthesis, biological evaluation, molecular docking and theoretical evaluation of ADMET properties of nepodin and chrysophanol derivatives as potential cyclooxygenase (COX-1, COX-2) inhibitors.[Pubmed: 24763362]
Nepodin and chrysophanol, isolated from Rumex nepalensis roots, showed significant cyclooxygenase (COX) inhibitory activity. To further optimize these lead molecules and study structure activity relationship (SAR), eighteen derivatives of nepodin and nine derivatives of chrysophanol were synthesized and evaluated for COX-1 and COX-2 inhibitory potential. Among the synthesized compounds, four nepodin (1f, 1g, 1h and 1i) and three chrysophanol (2e, 2f and 2h) derivatives displayed more pronounced COX-2 inhibition than their respective lead molecule. Further, compounds 1f, 1g, 2e and 2h exhibited better anti-inflammatory activity than ibuprofen in carrageenan-induced rat paw edema assay. Taking into account the in vitro and in vivo results, molecular docking and in silico prediction of ADMET properties of compounds were carried out respectively.
Anthraquinone derivatives from Rumex plants and endophytic Aspergillus fumigatus and their effects on diabetic nephropathy.[Pubmed: 23683594]
Two new oxanthrone C-glycosides, patientosides A (14) and B (15), together with three known ones (11-13), were isolated from Rumex patientia. Their structures were identified on the basis of spectroscopic methods. The absolute configuration for 14 and 15 were deduced by analysis of their CD spectra and comparison with those of known similar compounds. Compounds 11-15, and 14 known anthraquinones (1-4, 6-10, 16-20) previously isolated from Rumex nepalensis, Rumex hastatus, and endophytic Aspergillus fumigatus, respectively, as well as a commercially available compound rhein (5) were evaluated for their inhibitory effects on IL-6 and extracellular matrix production in mesangial cells.
Simultaneous determination of naphthalene and anthraquinone derivatives in Rumex nepalensis Spreng. roots by HPLC: comparison of different extraction methods and validation.[Pubmed: 21046683]
Rumex nepalensis contains mainly anthraquinone and naphthalene derivatives. Although HPLC methods have been reported for the analysis of anthraquinones, neither a phytochemical analysis of Rumex species nor the simultaneous determination of anthraquinone and naphthalene derivatives in other samples has been reported so far.
Anti-inflammatory, cyclooxygenase (COX)-2, COX-1 inhibitory, and free radical scavenging effects of Rumex nepalensis.[Pubmed: 20379952]
Evaluation of the topical anti-inflammatory activity of chloroform and ethyl acetate extracts of RUMEX NEPALENSIS roots in a TPA-induced acute inflammation mouse model demonstrated a significant reduction in ear edema. The extracts were further tested on purified enzymes for COX-1 and COX-2 inhibition to elucidate their mechanism of action, and a strong inhibition was observed. Six anthraquinones and two naphthalene derivatives were isolated from the ethyl acetate extract. Among the isolated compounds, emodin was found to be a potent inhibitor with slight selectivity towards COX-2, and nepodin exhibited selectivity towards COX-1. Emodin, endocrocin, and nepodin also exhibited significant topical anti-inflammatory activity in mice. Interestingly, nepodin showed better radical scavenging activity than trolox and ascorbic acid against DPPH and ABTS radicals. The strong radical scavenging activity of chloroform and ethyl acetate extracts could be explained by the presence of nepodin as well as by the high phenolic content of the ethyl acetate extract. Thus, the anti-inflammatory effect of R. NEPALENSIS roots was assumed to be mediated through COX inhibition by anthraquinones and naphthalene derivatives and through the radical scavenging activities of naphthalene derivatives.
New seco-anthraquinone glucosides from Rumex nepalensis.[Pubmed: 19291613]
Investigation of the N-BuOH extract of the roots of Rumex nepalensis afforded two new seco-anthraquinone glucosides, nepalensides A and B, along with nine known compounds, torachrysone ( 3), rumexoside, orientaloside, orcinol glucoside, aloesin, lyoniresinol 3 alpha- O- beta- D-glucopyranoside, (-)-epicatechin-3- O-gallate, (3,5-dimethoxy-4-hydroxyphenol)-1- O- beta- D-(6- O-galloyl) glucose, and (-)-epicatechin. Their structures were elucidated on the basis of spectroscopic methods. The possible formation of nepalensides A and B is briefly discussed.


