Peperomia tetraphylla
Peperomia tetraphylla
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Peperomia tetraphylla
- Cat.No. Product Name CAS Number COA
-
BCN3837
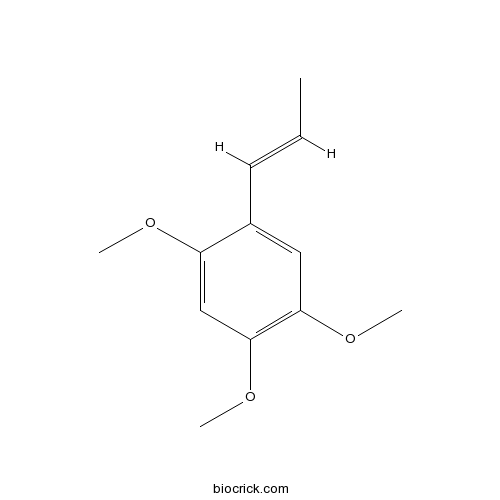
alpha-Asarone2883-98-9
Instructions
The natural phenolic peperobtusin A induces apoptosis of lymphoma U937 cells via the Caspase dependent and p38 MAPK signaling pathways.[Pubmed: 29604597]
Our previous research found the ethyl acetate extract of Peperomia tetraphylla (EAEPT) inhibited the growth of U937 cells by blocking the cell cycle and prompted apoptosis via the reactive oxygen species (ROS)-medicated mitochondria pathway. While the compounds in EAEPT which possessed the anti-tumor activity were unclear. Peperobtusin A is a phenolic compound, which was isolated from the whole plant of Peperomia tetraphylla. In this work, we found that peperobtusin A had the anti-proliferative effects against human lymphoma U937 cells and induced apoptosis in a dose dependent manner. Peperobtusin A significantly enhanced the formation of intracellular ROS and induced the loss of mitochondrial membrane potential (Δψm). And peperobtusin A could increase the ratio of Bax/Bcl-2, induce the cleavage of Bid, Caspase-3, Caspase-8 and Caspase-9 and enhance the level of P-P38. Moreover, peperobtusin A induced the accumulation of cells at S phase. Through using of inhibitors such as antioxidant NAC, pan-caspase inhibitor Z-VAD-FMK, p38 MAPK specific inhibitor SB203580, we found that intracellular ROS generation, activation of Caspases and p38 MAPK played very important roles in the apoptosis induced by peperobtusin A in U937 cells. Our results indicated that intracellular ROS generation, the Caspase-dependent and p38 MAPK signaling pathways involved in apoptosis induced by peperobtusin A in U937 cells.
Ethyl acetate extract of Peperomia tetraphylla induces cytotoxicity, cell cycle arrest, and apoptosis in lymphoma U937 cells.[Pubmed: 27847202]
The current study evaluated the cytotoxicity and the mechanism of apoptotic induction by Peperomia tetraphylla in U937 lymphoma cells. The results showed that P. tetraphylla ethyl acetate extract (EAEPT) inhibited the cell growth in U937 cells by MTT assay. After the U937 cells were treated with EAEPT, the cells exhibited marked morphological features of apoptosis (Hoechst 33342 staining) and the number of apoptotic cell (Annexin V-FITC/PI staining) increased. The treatment of EAEPT could induce loss of mitochondrial membrane potential (MMP) and increase the ROS level. Moreover, EAEPT treatment resulted in the accumulation of cells at S phase. We found that EAEPT could induce the cleavage of the caspase 3, caspase 8, caspase 9 and Bid. And the treatment of EAEPT could increase expression of Bax and down-regulate the expression of CCNB1, CCND1 and CDK1. The sub-fraction of EAEPT, namely EASub1 demonstrated the highest cytotoxicity activity on U937 cells. It was confirmed that EAEPT could inhibit the growth of U937 cells by blocking the cell cycle and prompted apoptosis via the ROS-medicated mitochondria pathway in vitro.
Peperotetraphin inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and apoptosis.[Pubmed: 25579167]
Peperotetraphin (methyl rel-(1R,2S,3S)-2,3-bis(7-methoxy-1,3-benzodioxol-5-yl) cyclobutanecarboxylate) was a novel cyclobutane-type norlignan, which was isolated from the whole plant of Peperomia tetraphylla. In this study, we explored its anti-tumor effect and the molecular mechanism in human prostate cancer PC-3 cell lines. Firstly, cell viability was evaluated by Cell Counting Kit (CCK-8) assay. The PC-3 cells were treated with increasing concentrations of peperotetraphin for 24, 48 and 72 h, respectively. The results showed that peperotetraphin inhibited the growth of PC-3 cell in a dose- and time-dependent manner. Next, the cell cycle distributions were analyzed by flow cytometric analysis (FCM), and the data suggested that peperotetraphin could significantly induce cell cycle arrested at the G1-S phase transition. Then, the cell apoptosis was detected by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and annexin V-FITC/PI dual staining analysis, the data confirmed apoptosis-inducing activity of peperotetraphin and the apoptosis rates increased from 3.9 to 32.3 % when treated with increasing concentrations of peperotetraphin from 0 to 50 µM. The expression levels of apoptosis-regulating protein caspase-3, Bax and Bcl-2 were also analyzed by Western blot analysis. The results showed that the expression levels of Bax and the activity of caspase-3 were upregulated, whereas the expression levels of Bcl-2 were downregulated compared with those of the control. These findings demonstrated that peperotetraphin exhibited effective cell growth inhibition by inducing cancer to undergo G1 phase arrest and apoptosis. The results suggested that peperotetraphin might have potential as chemoprevention or anti-tumor agent to prostatic cancer.
A new phenylpropanoid from Peperomia tetraphylla.[Pubmed: 22799497]
A new phenylpropanoid, (+)-methyl 3-acetoxy-3-(7-methoxy-1,3-benzodioxol-5-yl) propanoate (1), was obtained from the 95% EtOH extract of entire plant of Peperomia tetraphylla. The structure was elucidated based on the spectral analysis (IR, 1-D and 2-D NMR and HRESIMS). The absolute configuration of 1 was determined as (3R) by referring to analogous compound's optical rotation. Cytotoxicity assays showed that compound 1 had a moderate inhibitory activity against the A549 cell line, weak inhibitory activity against the Hela and the HepG2 cell lines.
Two New Norlignans and a New Lignanamide from Peperomia tetraphylla.[Pubmed: 22492494]
Two new cyclobutane-type norlignans, methyl rel-(1R,2S,3S)-2-(7-methoxy-1,3-benzodioxol-5-yl)-3-(2,4,5-trimethoxyphenyl)cyclobutanecarboxylate (1), and methyl rel-(1R,2R,3S)-2-(7-methoxy-1,3-benzodioxol-5-yl)-3-(2,4,5-trimethoxyphenyl)cyclobutanecarboxylate (2), and a new lignanamide, 3-hydroxy-N-[2-(4-hydroxyphenyl)ethyl]-α-[4-(2-{N-[2-(4-hydroxyphenyl)ethyl]carbamoyl}ethenyl)-3-methoxyphenoxy]-4-methoxycinnamamide 4,8″-ether (3), along with five known amides, 4-8, were obtained from the whole plant of Peperomia tetraphylla. Their structures were elucidated mainly by the analysis of NMR and MS data. The new compounds 1-3 and the known compound 4 were tested for their cytotoxic activities against the HepG2 (human hepatocarcinoma), A549 (human lung cancer), and HeLa (human cervical cancer) cell lines. Compound 4 showed significant cytotoxicity against HepG2 cell lines with an IC(50) value of 9.4 ± 1.0 μM.
[Amides from Peperomia tetraphylla].[Pubmed: 20450045]
To investigate the chemical constituents of Peperomia tetraphylla.


