Glycyrrhiza inflata
Glycyrrhiza inflata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Glycyrrhiza inflata
- Cat.No. Product Name CAS Number COA
-
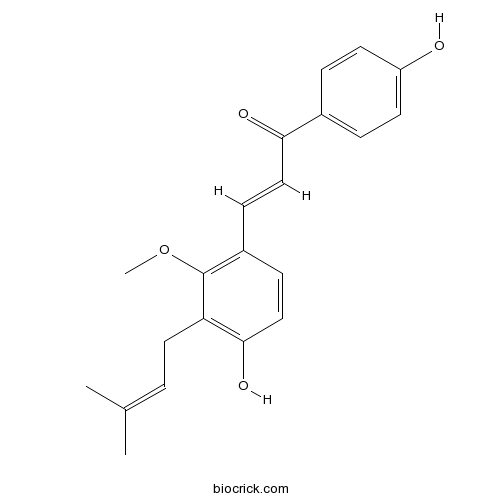
BCN6334
Licochalcone C144506-14-9
Instructions
-
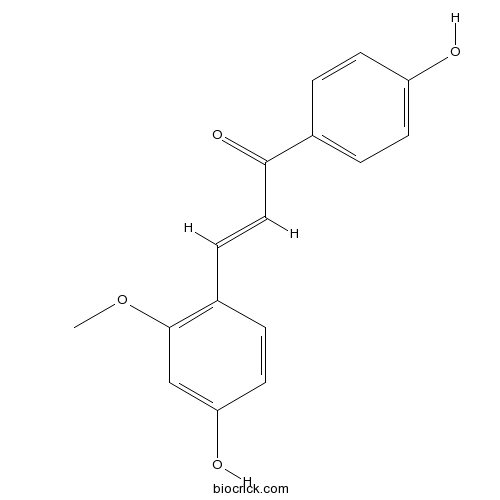
BCN6277
Echinatin34221-41-5
Instructions
-
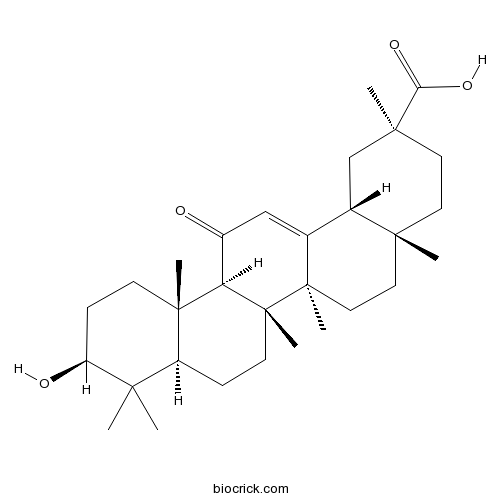
BCN5942
Glycyrrhetinic acid471-53-4
Instructions
-
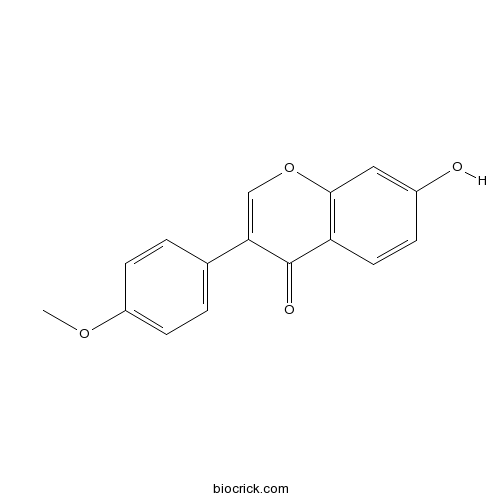
BCN1061
Formononetin485-72-3
Instructions
-
BCN5946
Liquiritigenin578-86-9
Instructions
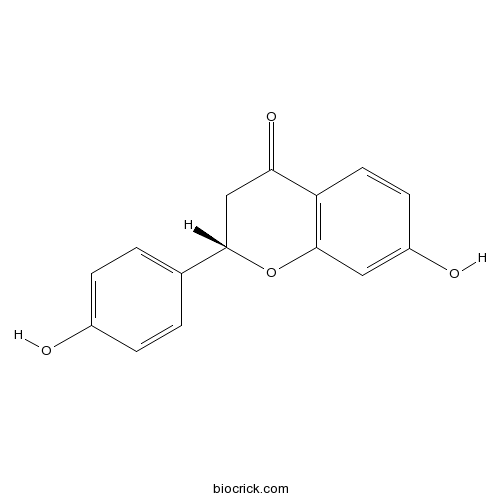
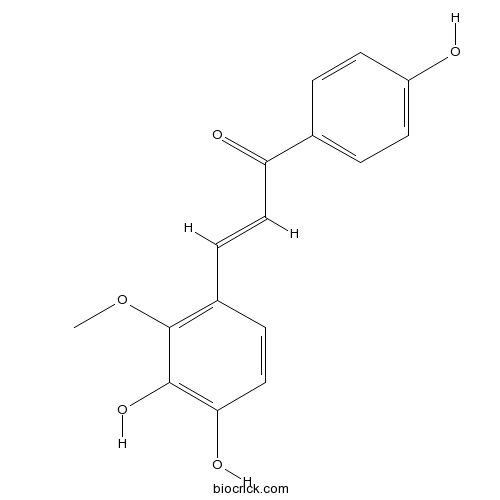
-
BCN6333
Licochalcone B58749-23-8
Instructions
Actinophytocola glycyrrhizae sp. nov. isolated from the rhizosphere of Glycyrrhiza inflata.[Pubmed: 29939121]
None
Dihydroisocoumarins from Radix Glycyrrhizae.[Pubmed: 29748827]
Radix Glycyrrhizae is the rhizome of Glycyrrhiza inflata Bat., Glycyrrhiza uralensis Fisch. or Glycyrrhiza glabra L. The present paper describes the isolation and the structural elucidation of three new dihydroisocoumarins obtained from the 70% EtOH extract of Radix Glycyrrhizae. And the cytotoxic activities of these new compounds were also evaluated using four cell lines, subsequently.
Estrogen Receptor (ER) Subtype Selectivity Identifies 8-Prenylapigenin as an ERβ Agonist from Glycyrrhiza inflata and Highlights the Importance of Chemical and Biological Authentication.[Pubmed: 29641206]
Postmenopausal women are increasingly using botanicals for menopausal symptom relief due to the increased breast cancer risk associated with traditional estrogen therapy. The deleterious effects of estrogens are associated with estrogen receptor (ER)α-dependent proliferation, while ERβ activation could enhance safety by opposing ERα effects. Three medicinal licorice species, Glycyrrhiza glabra ( G. glabra), G. uralensis, and G. inflata, were studied for their differential estrogenic efficacy. The data showed higher estrogenic potency for G. inflata in an alkaline phosphatase induction assay in Ishikawa cells (ERα) and an estrogen responsive element (ERE)-luciferase assay in MDA-MB-231/β41 breast cancer cells (ERβ). Bioassay-guided fractionation of G. inflata led to the isolation of 8-prenylapigenin (3). Surprisingly, a commercial batch of 3 was devoid of estrogenic activity. Quality control by MS and qNMR revealed an incorrect compound, 4'- O-methylbroussochalcone B (10), illustrating the importance of both structural and purity verification prior to any biological investigations. Authentic and pure 3 displayed 14-fold preferential ERβ agonist activity. Quantitative analyses revealed that 3 was 33 times more concentrated in G. inflata compared to the other medicinal licorice extracts. These data suggest that standardization of G. inflata to 3 might enhance the safety and efficacy of G. inflata supplements used for postmenopausal women's health.
The genetic and chemical diversity in three original plants of licorice, Glycyrriza uralensis Fisch., Glycyrrhiza inflata Bat. and Glycyrrhiza glabra L.[Pubmed: 29618444]
None
Hepatic metabolism of licochalcone A, a potential chemopreventive chalcone from licorice (Glycyrrhiza inflata), determined using liquid chromatography-tandem mass spectrometry.[Pubmed: 29127460]
The metabolism of the chemoprevention agent licochalcone A, which is a chemopreventive chalcone found in abundance in the licorice species Glycyrrhiza inflata, was investigated using human liver microsomes and human hepatocytes combined with analysis using high performance liquid chromatography-mass spectrometry (LC-MS). Five oxygenated phase I metabolites of licochalcone A were formed by human liver microsomes, including a catechol on the A-ring, two intramolecular cyclization products following epoxidation of the exocyclic alkene at position 5 of the B-ring, and two dioxygenated products. Nine phase II monoglucuronides of licochalcone A and its oxygenated phase I metabolites were formed during incubation with human hepatocytes. These included (E)-licochalcone A-4-glucuronide, (E)-licochalcone A-4'-glucuronide, (Z)-licochalcone A-4-glucuronide, glucuronic acid conjugates of all of the monooxygenated phase I metabolites, and glucuronides of the licochalcone catechol after methylation by catechol-O-methyl transferase. In addition, human hepatocytes formed one sulfate conjugate and one glutathione conjugate of licochalcone A. The structures of all major metabolites were determined using a combination of accurate mass measurement, LC-tandem mass spectrometry, LC-UV, nuclear magnetic resonance, and comparison with standards. The cytochrome P450 enzymes and UDP-glucuronosyltransferases responsible for the formation of the major metabolites were identified. Based on in vitro hepatic clearance calculations, licochalcone A is predicted to be metabolized primarily by phase II conjugation reactions. Graphical abstract Phase I and II metabolism of licochalcone A from the licorice species Glycyrrhiza inflata by human liver microsomes and hepatocytes determined using LC-MS/MS, LC-UV and NMR.


