Daphne aurantiaca
Daphne aurantiaca
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Daphne aurantiaca
- Cat.No. Product Name CAS Number COA
-
BCN4985
Luteolin-6-C-glucoside4261-42-1
Instructions
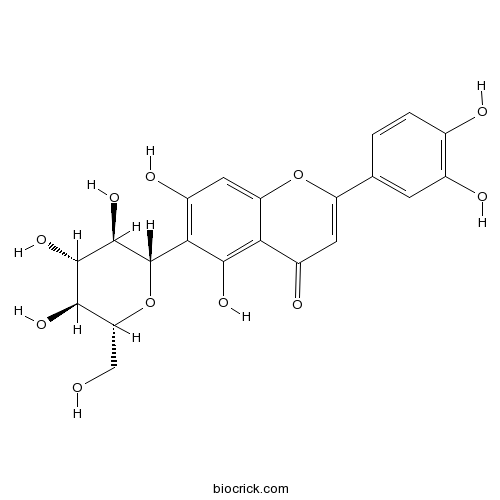
-
BCN5488
Genkwanin437-64-9
Instructions
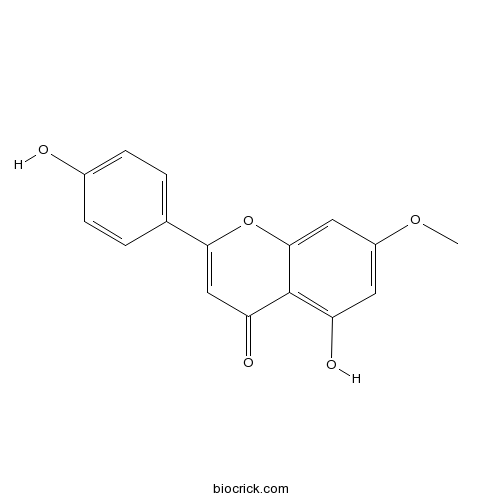
Daphnauranins A and B, two new antifeedants Isolated from Daphne aurantiaca roots.[Pubmed: 28821449]
Daphnauranins A (1) and B (2), two sesquiterpenoids were isolated from the roots of Daphne aurantiaca Diels. One is an unprecedented 5/7 oxacycloheptane ring system, the other is a sesquiterpene-lignan complex. Their structures were elucidated by comprehensive spectroscopic methods including MS and NMR. Their absolute configurations were further confirmed by the quantum ECD calculations. Daphnauranins A and B showed anti-insect activities against male fruit fly with anti-feeding rate up to 46.2±7.1 and 44.7±5.4% at 1mM, respectively.
Two novel innovanoside dimers from Daphne aurantiaca and a concise total synthesis of diinnovanoside A.[Pubmed: 23687654]
Chemical examination of the methanolic extract from the stem bark of Daphne aurantiaca led to the isolation of two innovanoside dimers (1 and 2) with an unusual four-membered cyclobutane ring, together with the isoinnovanoside 3. Their chemical structures and configurations were elucidated by extensive spectral analysis and synthesis.
Two new daucane sesquiterpenoids from Daphne aurantiaca.[Pubmed: 22922275]
Two new daucane sesquiterpenoids 1β,2β-epoxy-10(H)α-dauca-11(12)-ene-7α,14-diol and 1α,2α-epoxy-10(H)α-dauca-11(12)-ene-7α,14-diol were isolated from the plateau medicinal plant Daphne aurantiaca Diels. (Thymelaeceae). Their structures were elucidated by 1D and 2D NMR spectroscopy, as well as HR-ESI-MS data.
Flavonoids from Daphne aurantiaca and their inhibitory activities against nitric oxide production.[Pubmed: 21532205]
Chemical examination of the methanolic extract from the stem bark of Daphne aurantiaca led to the isolation of three new flavonoids (1-3), and 29 known flavonoids. All 32 compounds were isolated for the first time from Daphne aurantiaca. The isolates were tested for inhibitory activities against lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW 264.7 macrophages. Compounds 21 and 24 showed potent inhibitory activities against the production of NO with IC₅₀ values of 0.006 and 0.076 µM, respectively.
Terpenoids from Daphne aurantiaca and their potential anti-inflammatory activity.[Pubmed: 20192236]
Phytochemical examination of the methanolic extract from the stem bark of Daphne aurantiaca led to the isolation of six new sesquiterpenoids, dauca-3,11-dien-2alpha,15-diol (1), 3-oxoguai-4-ene-11,12-diol (2), 4alpha,5alpha,8alpha,11alphaH-3-oxoguai-1(10)-en-12,8-olide-7alpha-diol (3), 4alpha,5alpha,8alpha,11betaH-3-oxoguai-1(10)-en-12,8-olide-7beta-diol (4), 4alpha,5betaH-guai-9,7(11)-dien-12,8-olide-1alpha,8alpha-diol (5), 4alpha,5alphaH-guai-9,7(11)-dien-12,8-olide-1alpha,8alpha-diol (6), and a new diterpenoid, 12-O-benzoylphorbol 13-nonanoate (7), together with 10 known terpenoids. All compounds were tested for inhibitory activity against LPS-induced NO production in RAW 264.7 macrophages. Compounds 7, 8, 9, 10, and 11 showed potent inhibitory activities against NO production with IC(50) values of 0.01, 0.01, 0.06, 0.07, and 0.03 microM, respectively.


