Caesalpinia millettii
Caesalpinia millettii
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Caesalpinia millettii
- Cat.No. Product Name CAS Number COA
-
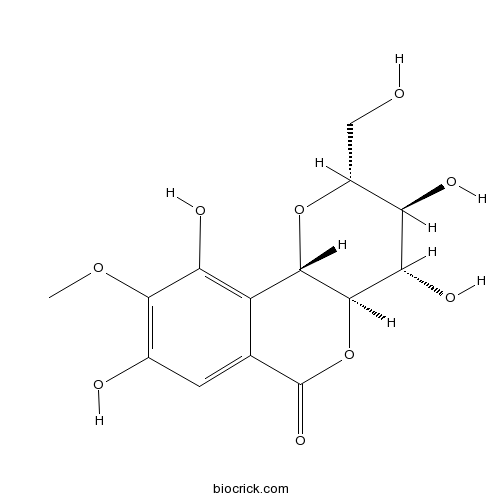
BCN5540
Bergenin477-90-7
Instructions
-
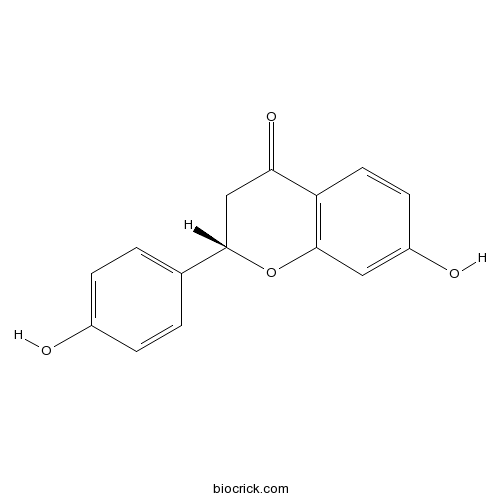
BCN5946
Liquiritigenin578-86-9
Instructions
-
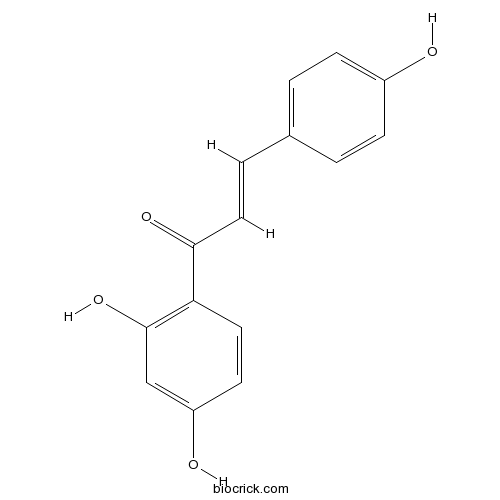
BCN4512
Isoliquiritigenin961-29-5
Instructions
[Chemical constituents and antibacterial activity contained in Caesalpinia millettii].[Pubmed: 23126193]
To study chemical constituents contained in roots of Caesalpinia millettii by HPLC. Six homoisoflavonoids were identified by spectroscopic data and physicochemical property as eucomin (1), intricatinol (2), 8-methoxybonducellin (3), bonducellin (4), 8-methoxyisobonducellin (5) and 3-(4-methoxybenzyl) -5, 7-dimethoxychroman-4-one (6). All compounds were separated from the root of this genus for the first time. An antibacterial screening was made on eight monomeric compounds. Among them, 8-methoxyisobonducellin, intricatinol, bergenin, hyperoside and 11-O-galloylbergenin showed a inhibitory effect on Staphylococcus aureus, Klebsiella Peneumoniae, Beta streptococcus and Aeruginosus bacillus.
Flavonol galactoside caffeiate ester and homoisoflavones from Caesalpinia millettii HOOK. et ARN.[Pubmed: 17409566]
Chemical examination of the stems of Caesalpinia millettii HOOK. et ARN. led to the isolation of new flavonol glycoside caffeiate ester (1) and homoisoflavone (2), along with four known homoisoflavones: eucomin (3), bonducellin (4), 8-methoxybonducellin (5) and intricatinol (6). The structures of 1 and 2 were established to be tamarixetin 3-O-(6''-O-E-caffeoyl)-beta-D-galactopyranoside (1) and (Z)-7-hydroxy-8-methoxy-3-(4-methoxybenzyl) chroman-4-one (2) on the basis of detailed analyses of physical, chemical, and spectral data. Compounds 3-6 were isolated from this plant for the first time.


