Asparagus officinalis
Asparagus officinalis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Asparagus officinalis
- Cat.No. Product Name CAS Number COA
-
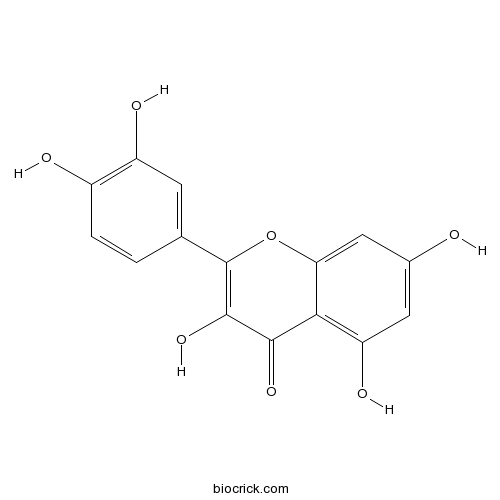
BCN6049
Quercetin117-39-5
Instructions
-
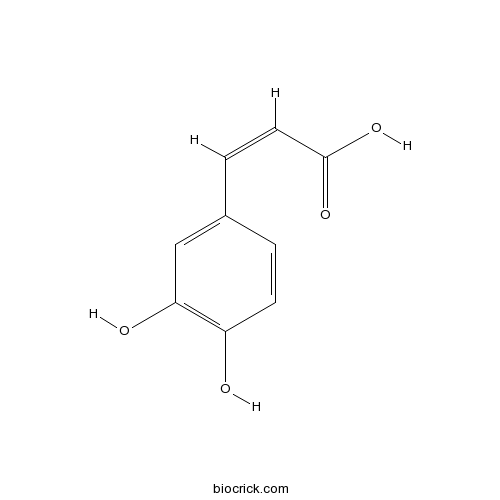
BCN5979
Caffeic acid331-39-5
Instructions
-
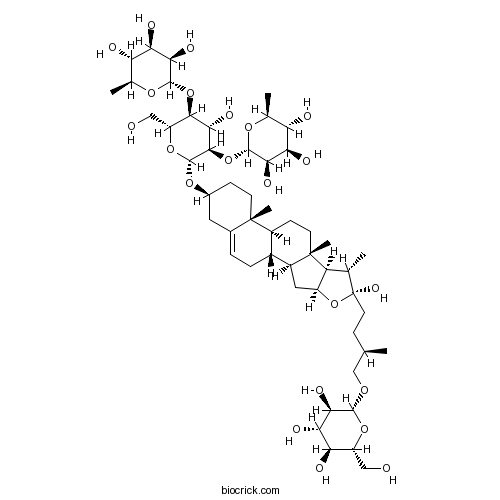
BCN6274
Protodioscin55056-80-9
Instructions
Purification, Characterization, and Functional Analysis of a novel 6G&1-FEH mainly Hydrolyzing Neokestose from Asparagus.[Pubmed: 29931209]
Asparagus (Asparagus officinalis L.) accumulates inulin- and inulin neoseries-type fructans. Fructose released by the hydrolysis of fructans is an energy source for emerging asparagus spears. Plant fructans are hydrolyzed by fructan exohydrolases (FEH), whose presence in asparagus has not yet been fully characterized. Here, we describe for the first time the purification and characterization of a FEH from asparagus, and the functional analysis of its gene. The purified enzyme was predicted to exist as a dimer (approximately 130 kDa), consisting of two polypeptides with a molecular mass of approximately 68 kDa. N-terminal sequences of the purified enzyme were matched with the amino acid sequences of aoeh4a and aoeh4b-cDNAs isolated from asparagus (cv. Gijnlim and Taihouwase). Native enzymes obtained from asparagus roots and recombinant enzymes produced by Pichia pastoris showed fructan 1-exohydrolase (1-FEH) activity via the hydrolysis of inulin-type fructan. Unlike other 1-FEHs, these enzymes showed minimal hydrolysis of 1-kestose but efficiently hydrolyzed neokestose. Therefore, the enzyme was termed 6G&1-FEH. Gene expression studies in asparagus roots showed that aoeh4 increased during low-temperature periods and harvesting. These findings suggest that 6G&1-FEH described here may be involved in fructan hydrolysis in asparagus roots for emerging asparagus spears.


