Aralia continentalis
Aralia continentalis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Aralia continentalis
- Cat.No. Product Name CAS Number COA
-
BCN4600
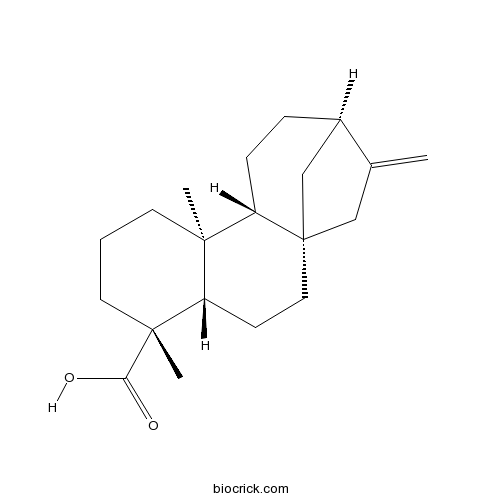
Kaurenoic acid6730-83-2
Instructions
Anti-inflammatory and anti-arthritic effects of the ethanolic extract of Aralia continentalis Kitag. in IL-1β-stimulated human fibroblast-like synoviocytes and rodent models of polyarthritis and nociception.[Pubmed: 29425654]
Blocking the formation and invasive growth of pannus and its secretion of inflammatory cytokines and MMPs is important for treating rheumatoid arthritis.
Kaurenoic acid activates TGF-β signaling.[Pubmed: 28732811]
Kaurenoic acid (ent-kaur-16-en-19-oic acid: KA) is a key constituent found in the roots of Aralia continentalis Kitagawa (Araliaceae) that has been used for treating rheumatism in traditional Asian medicine.
Ultra-performance convergence chromatography for the quantitative determination of bioactive compounds in Aralia continentalis Kitagawa as quality control markers.[Pubmed: 28306202]
None
Therapeutic effect of ent-kaur-16-en-19-oic acid on neutrophilic lung inflammation and sepsis is mediated by Nrf2.[Pubmed: 27133718]
Kaurenoic acid (ent-kaur-16-en-19-oic acid: KA) is a key constituent found in the roots of Aralia continentalis Kitagawa (Araliaceae), a remedy to treat patients with inflammatory diseases in traditional Asian medicine. Since KA activates Nrf2, a key anti-inflammatory factor, at the cellular level, we explored a possible therapeutic usage of KA against neutrophilic inflammatory lung disease such as acute lung injury (ALI). Intraperitoneal (i.p.) injection of lipopolysaccharide (LPS) to C57BL/6 mice induced lung inflammation as in ALI. 2 h after i.p. LPS, intratracheal (i.t.) delivery of KA (0.3, 3, or 30 μg/kg body weight) improved lung structure and significantly suppressed neutrophil infiltrations to mouse lungs, with concomitant reduction of myeloperoxidase activity and of the expression of pro-inflammatory cytokine genes. While activating Nrf2 and expressing Nrf2-dependent genes in mouse lungs, KA did not significantly suppress neutrophil lung inflammation in Nrf2 KO mice. In a mouse model of sepsis, a major cause of ALI, single i.t. KA (3 μg/kg) 2 h after the onset of sepsis significantly decreased the mortality of mice. Together, these results suggest that KA has a therapeutic potential against inflammatory lung disease, the effect of which is associated with Nrf2 activation.
Rapid Authentication of the Herbal Medicine Plant Species Aralia continentalis Kitag. and Angelica biserrata C.Q. Yuan and R.H. Shan Using ITS2 Sequences and Multiplex-SCAR Markers.[Pubmed: 26938512]
Accurate identification of the plant species that are present in herbal medicines is important for quality control. Although the dried roots of Aralia continentalis (Araliae Continentalis Radix) and Angelica biserrata (Angelicae Pubescentis Radix) are used in the same traditional medicine, namely Dok-Hwal in Korean and Du-Huo in Chinese, the medicines are described differently in the national pharmacopeia. Further confusion arises from the distribution of dried Levisticum officinale and Heracleum moellendorffii roots as the same medicine. Medicinal ingredients from all four plants are morphologically similar, and discrimination is difficult using conventional methods. Molecular identification methods offer rapidity and accuracy. The internal transcribed spacer 2 (ITS2) region of the nuclear ribosomal RNA gene (rDNA) was sequenced in all four plant species, and the sequences were used to design species-specific primers. Primers for each species were then combined to allow sample analysis in a single PCR reaction. Commercial herbal medicine samples were obtained from Korea and China and analyzed using the multiplex assay. The assay successfully identified authentic medicines and also identified inauthentic or adulterated samples. The multiplex assay will be a useful tool for identification of authentic Araliae Continentalis Radix and/or Angelicae Pubescentis Radix preparations in Korea and China.
A strategy for the separation of diterpenoid isomers from the root of Aralia continentalis by countercurrent chromatography: The distribution ratio as a substitute for the partition coefficient and a three-phase solvent system.[Pubmed: 26138601]
Aralia continentalis (Araliaceae) is widely used as a medicinal plant in East Asia. Previous studies have indicated that diterpenoid isomers (kaurenoic acid, continentalic acid, and ent-continentalic acid) are the major bioactive compounds of this plant. A new strategy was developed to alleviate difficulties in the separation of these isomers from this plant. A three-phase solvent system was applied to separate the isomers, and furthermore, the distribution ratio (Kc) was introduced as a substitute for the partition coefficient (KD). For compounds exhibiting a single equilibrium, their distributions in two immiscible phases were only affected by the partition coefficient of each solute. However, compounds that have a dissociating functional group (e.g., -COOH) are involved in two types of equilibrium in the two-phase system. In this case, the partitioning behaviors of the solutes are greatly affected by the pH of the solution. A mathematical prediction was applied for adjusting the solutions to the proper pH values. To prevent non-used phase (medium phase) waste, both the stationary phase (upper phase) and mobile phase (lower phase) were prepared on-demand without pre-saturation with the application of (1)H NMR. Each fraction obtained was collected and dried, yielding the following diterpenoid isomers from the 50mg injected sample: kaurenoic acid (19.7mg, yield: 39%) and ent-continentalic acid (21.3mg, yield: 42%).
HY253, a novel decahydrofluorene analog, induces apoptosis via intrinsic pathway and cell cycle arrest in liver cancer HepG2 cells.[Pubmed: 25674801]
Recently, we isolated HY253, a novel decahydrofluorene analog with a molecular structure of 7,8a-divinyl-2,4a,4b,5,6,7,8,8a,9,9a-decahydro-1H-fluorene-2,4a,4b,9a-tetraol from the roots of Aralia continentalis, which is known as Dokwhal, a traditional medicinal herb. Moreover, we previously reported its cytotoxic activity on cancer cell proliferation in human lung cancer A549 and cervical cancer HeLa cells. The current study aimed to evaluate its detailed molecular mechanisms in cell cycle arrest and apoptotic induction in human hepatocellular carcinoma HepG2 cells. Flow cytometric analysis of HepG2 cells treated with 60 micrometer HY253 revealed appreciable cell cycle arrest at the G1 phase via inhibition of Rb phosphorylation and down-regulation of cyclin D1. Furthermore, using western blots, we found that up-regulation of cyclin-dependent kinase inhibitors, such as p21(CIP1) and p27(KIP1), was associated with this G1 phase arrest. Moreover, TUNEL assay and immunoblottings revealed apoptotic induction in HepG2 cells treated with 100 micrometer HY253 for 24 h, which is associated with cytochrome c release from mitochondria, via down-regulation of anti-apoptotic Bcl-2 protein, which in turn resulted in activation of caspase-9 and -3, and proteolytic cleavage of poly(ADP-ribose) polymerase (PARP). Accordingly, we suggest that HY253 may be a potent chemotherapeutic hit compound for treating human liver cancer cells via up-regulation and activation of the p53 gene.
Optimization of ultrasonic-assisted extraction of continentalic acid from the root of Aralia continentalis by using the response surface methodology.[Pubmed: 24293061]
In order to optimize the extraction conditions of continentalic acid, the main compound from Aralia continentalis root, we developed a model using the response surface methodology. The continentalic acid yield was analyzed and quantified by high-performance liquid chromatography coupled with UV detection. The extraction solvent, temperature, and time the three main factors for ultrasound-assisted extraction were optimized using the central composite design. Analysis of variance showed a good model fit (R (2) = 0.9323). The maximum extraction of continentalic acid obtained experimentally was 1.0138 % under an extraction temperature of 33 °C and extraction time of 28 min when 100 % ethanol was used as the solvent. The experimental value was in good agreement (the yield 1.0103 %) with those predicted values. The results clearly showed that quality by statistical design could be effectively applied to optimize extraction of continentalic acid.
Kaurenoic Acid from Aralia continentalis Inhibits Biofilm Formation of Streptococcus mutans.[Pubmed: 23662113]
We isolated a single chemical compound from A. continentalis and identified it to be kaurenoic acid (KA) and investigated the influence of anticariogenic properties. Inhibitory effects of KA on cariogenic properties such as growth, acid production, biofilm formation, and the adherence of S. mutans were evaluated. Furthermore, real-time PCR analysis was performed to evaluate the influence of KA on the genetic expression of virulence factors. KA significantly inhibited the growth and acid production of S. mutans at 2-4 μ g/mL and 4 μ g/mL of KA, respectively. Furthermore, the adherence onto S-HAs was inhibited at 3-4 μ g/mL of KA and biofilm formation was significantly inhibited when treated with 3 μ g/mL KA and completely inhibited at 4 μ g/mL. Also, the inhibitory effect of KA on biofilm formation was confirmed by SEM. In confocal laser scanning microscopy, bacterial viability gradually decreased by KA in a dose dependent manner. Real-time PCR analysis showed that the expressions of gtfB, gtfC, gbpB, spaP, brpA, relA, and vicR were significantly decreased in S. mutans when it was treated with KA. These results suggest that KA from A. continentalis may be a useful agent for inhibiting the cariogenic properties of S. mutans.


