Aconitum sinomontanum
Aconitum sinomontanum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Aconitum sinomontanum
- Cat.No. Product Name CAS Number COA
-
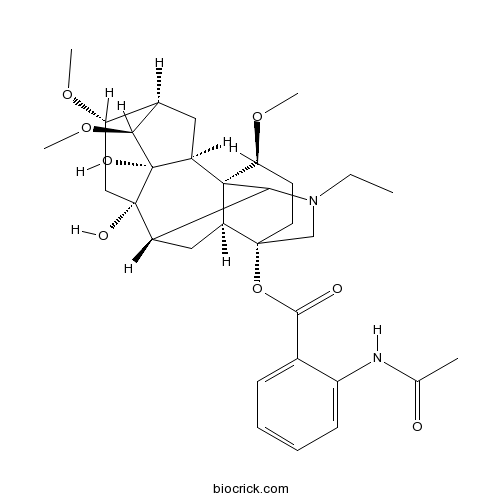
BCN2504
Lannaconitine32854-75-4
Instructions
Biomass-Based Carbon Nanofibers Prepared by Electrospinning for Supercapacitor.[Pubmed: 29458633]
Biomass-based carbon nanofibers were prepared by double-nozzle electrospinning the aqueous solution of acid treated the waste medicine Aconitum sinomontanum Nakai extraction and poly-acrylonitrile followed by thermal treatment in an inert atmosphere. The structural, constituent and surface properties of biomass-based carbon nanofibers were investigated by means of spectroscopic, microscopy, energy spectrometer and Brunauer-Emmet-Teller (BET) techniques. The results showed that the biomass-based carbon nanofibers had abundant pore structure and large specific surface area. The electrochemical performance of supercapacitor electrodes with the nanofibers was studied. This electrode showed a capacitance of 295 F/g at the current density of 1 A/g in 6 mol/L aqueous KOH electrolyte, and 98.5% capacity retention after 1000 charge/discharge cycles at the current density of 2 A/g. This indicate that the activate biomass-based carbon nanofibers have a good electrochemical stability.
Microneedle-mediated transdermal delivery of nanostructured lipid carriers for alkaloids from Aconitum sinomontanum.[Pubmed: 28899209]
A combination method using microneedle (MN) pretreatment and nanostructured lipid carriers (NLCs) was developed to improve the transdermal delivery of therapeutics. The MN treatment of the skin and co-administration of NLCs loaded with total alkaloids isolated from Aconitum sinomontanum (AAS-NLCs) significantly increased the skin permeation of the drugs. Fluorescence imaging confirmed that MNs could provide microchannels penetrating the stratum corneum, and delivery of NLCs through the channels led to their deeper permeation. In vivo studies showed that combination of AAS-NLCs with MNs (AAS-NLCs-MN) in transdermal delivery could improve the bioavailability and maintain stable drug concentrations in the blood. Moreover, AAS-NLCs-MN showed benefits in eliminating paw swelling, decreasing inflammation and pain, and regulating immune function in adjuvant arthritis rats. After administration of AAS-NLCs-MN, no skin irritation was observed in rabbits, and electrocardiograms of rats showed improved arrhythmia. These results indicated that the dual approach combining MN insertion and NLCs has the potential to provide safe transdermal delivery and to improve the therapeutic efficacy through sustained release of AAS.
Crystal structure of N-de-acetyl-lappa-coni-tine.[Pubmed: 26396805]
The title compound, C30H42N2O7 [systematic name: (1S,4S,5S,7S,8S,9S,10S,11S,13R,14S,16S,17R)-20-ethyl-4,8,9-trihy-droxy-1,14,16-tri-meth-oxy-aconitan-4-yl 2-amino-benzoate], isolated from roots of Aconitum sinomontanum Nakai, is a typical aconitane-type C19-diterpenoid alkaloid, which crystallizes with two independent mol-ecules in the asymmetric unit. The conformations of the two independent mol-ecules are closely similar. Each mol-ecule comprises four six-membered rings (A, B, D and E) including one six-membered N-containing heterocyclic ring (E), and two five-membered rings (C and F). Rings A, B and E adopt chair conformations, while ring D displays a boat conformation. Five-membered rings C and F exhibit envelope conformations. IntramolecularN-H⋯O hydrogen bonds between the amino group and carbonyl O atom help to stabilize molecular structure. In the crystal, O-H⋯O hydrogen bonds link the mol-ecules into zigzag chains propagating in [010].
Crystal structure of sepaconitine, a C19-diterpenoid alkaloid from the roots of Aconitum sinomontanum Nakai.[Pubmed: 26396791]
The title compound [systematic name: [(1α,14α,16β)-20-ethyl-8,9,10-trihy-droxy-1,14,16-tri-meth-oxy-aconitan-4-yl 2-amino-benzoate], C30H42N2O8, a natural C19-diterpenoid alkaloid, possesses an aconitane carbon skeleton with four six-membered rings and two five-membered rings. The fused ring system contains two chair, one boat, one twist-boat and two envelope conformations. Intra-molecular N-H⋯O hydrogen bonds are observed between the amino and carbonyl groups. The mol-ecules are linked together via O-H⋯O hydrogen bonds, forming a three-dimensional framework.
Nanostructured lipid carriers for percutaneous administration of alkaloids isolated from Aconitum sinomontanum.[Pubmed: 26156035]
Lipid-based nanosystems have great potential for transdermal drug delivery. In this study, nanostructured lipid carriers (NLCs) for short-acting alkaloids lappacontine (LA) and ranaconitine (RAN) isolated from Aconitum sinomontanum (AAS) at 69.47 and 9.16% (w/w) yields, respectively, were prepared to enhance percutaneous permeation. Optimized NLC formulations were evaluated using uniform design experiments. Microstructure and in vitro/in vivo transdermal delivery characteristics of AAS-loaded NLCs and solid lipid nanoparticles (SLNs) were compared. Cellular uptake of fluorescence-labeled nanoparticles was probed using laser scanning confocal microscopy and fluorescence-activated cell sorting. Nanoparticle integrity during transdermal delivery and effects on the skin surface were also investigated.
X-ray crystallographic study of ranaconitine.[Pubmed: 22224266]
The crystal structure of natural diterpenoid alkaloid ranaconitine isolated from Aconitum sinomontanum Nakai has been determined by single crystal X-ray diffraction analysis. The crystal presents a monoclinic system, space group C2 with Z = 4, unit cell dimensions a = 30.972(19) angstrom, b = 7.688(5) angstrom, and c = 19.632(12) angstrom. Moreover, the intermolecular O-H...O hydrogen bonds and weak pi-pi interactions play a critical role in expanding the dimensionality.
Isolation of active substances and bioactivity of Aconitum sinomontanum Nakai.[Pubmed: 22007953]
In order to obtain the active compounds against third armyworm (Mythimna separata) contained in Aconitum sinomontanum Nakai by solvent partition, successive fractionation on silica gel and bioassay by the conventional leaf disc method, three diterpenoidal alkaloids were isolated for the first time from the roots of A. sinomontanum Nakai. Their structures were established as demethylenedelcorine, 18-O-methylgigactonine and lepenine based on their spectral data and X-ray diffraction analysis. Demethylenedelcorine and 18-O-methylgigactonine exhibited better pesticidal activities against M. separata, while lepenine had the induce-feeding activity against M. separata. The three diterpenoidal alkaloids exhibited bactericidal and fungicidal activities against certain kinds of the tested bacteria and fungi.
X-ray structure study of ranaconitine hydrobromide.[Pubmed: 21988549]
Ranaconitine is an important diterpenoid alkaloid from Aconitum sinomontanum Nakai. The absolute configuration of natural ranaconitine was determined through an X-ray structure analysis of hydrated ranaconitine hydrobromide. The crystal presents a monoclinic system, space group P2(1) with Z = 2, unit cell dimensions: a = 10.6604(12) Å, b = 12.3674(14) Å, c = 12.2938(13) Å and β = 91.056(2)°. The chirality of the asymmetric carbon atoms was as follows: C10(S), C13(S), C14(S), C15(S), C16(S), C17(R), C23(S), C25(R), C26(S), C27(S), C28(S) and C30(S). Moreover, a complex network of hydrogen bonds occurred between neighbouring molecules.


