Z-Gly-OHCAS# 1138-80-3 |
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1138-80-3 | SDF | Download SDF |
| PubChem ID | 14349 | Appearance | Powder |
| Formula | C10H11NO4 | M.Wt | 209.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
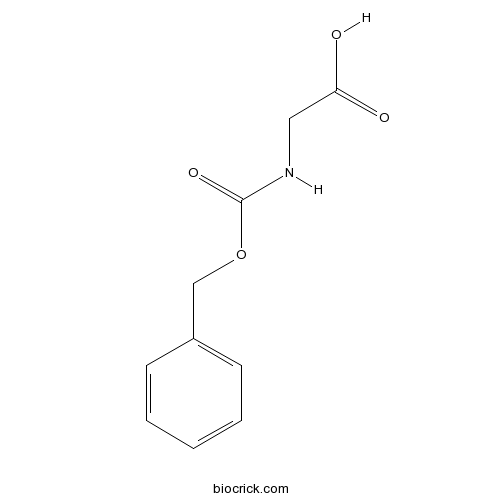
| Chemical Name | 2-(phenylmethoxycarbonylamino)acetic acid | ||
| SMILES | C1=CC=C(C=C1)COC(=O)NCC(=O)O | ||
| Standard InChIKey | CJUMAFVKTCBCJK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H11NO4/c12-9(13)6-11-10(14)15-7-8-4-2-1-3-5-8/h1-5H,6-7H2,(H,11,14)(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Gly-OH Dilution Calculator

Z-Gly-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7801 mL | 23.9006 mL | 47.8011 mL | 95.6023 mL | 119.5029 mL |
| 5 mM | 0.956 mL | 4.7801 mL | 9.5602 mL | 19.1205 mL | 23.9006 mL |
| 10 mM | 0.478 mL | 2.3901 mL | 4.7801 mL | 9.5602 mL | 11.9503 mL |
| 50 mM | 0.0956 mL | 0.478 mL | 0.956 mL | 1.912 mL | 2.3901 mL |
| 100 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.956 mL | 1.195 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-Gly-OH
- (Z)-FeCP-oxindole
Catalog No.:BCC6079
CAS No.:1137967-28-2
- TAK960
Catalog No.:BCC6411
CAS No.:1137868-52-0
- MDL 72832 hydrochloride
Catalog No.:BCC6637
CAS No.:113777-40-5
- Dexmedetomidine
Catalog No.:BCC4326
CAS No.:113775-47-6
- Eudesm-4(15)-ene-3alpha,11-diol
Catalog No.:BCN4060
CAS No.:113773-90-3
- Ilexhainanoside D
Catalog No.:BCN7863
CAS No.:1137648-52-2
- LX1606
Catalog No.:BCC1713
CAS No.:1137608-69-5
- BRD 7552
Catalog No.:BCC8035
CAS No.:1137359-47-7
- cis-Ned 19
Catalog No.:BCC6089
CAS No.:1137264-00-6
- Tenatoprazole
Catalog No.:BCC4732
CAS No.:113712-98-4
- BOC-D-ARG-OH.HCL.H2O
Catalog No.:BCC3069
CAS No.:113712-06-4
- 4-Aminobenzophenone
Catalog No.:BCC8684
CAS No.:1137-41-3
- Picrasidine T
Catalog No.:BCN6017
CAS No.:113808-03-0
- N1,N12-Diethylspermine tetrahydrochloride
Catalog No.:BCC6669
CAS No.:113812-15-0
- N-p-coumaroyl-N'-caffeoylputrescine
Catalog No.:BCN6018
CAS No.:1138156-77-0
- Cidofovir
Catalog No.:BCC2546
CAS No.:113852-37-2
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Cinnamyl 3-aminobut-2-enoate
Catalog No.:BCC8914
CAS No.:113898-97-8
- Caryophyllene oxide
Catalog No.:BCN6019
CAS No.:1139-30-6
- Koumine N-oxide
Catalog No.:BCN4807
CAS No.:113900-75-7
- PNU 74654
Catalog No.:BCC7704
CAS No.:113906-27-7
- PMPA (NMDA antagonist)
Catalog No.:BCC7308
CAS No.:113919-36-1
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
Peptide ionophores: synthesis and cation-binding properties of a bicyclic peptide containing glycine and lysine residues.[Pubmed:7655187]
Pept Res. 1995 Mar-Apr;8(2):62-9.
Peptide 1, cyclo(1,5-epsilon-succinoyl) (Lys-Gly-Gly-Gly)2, is a representative member of a family of polycyclic peptide ionophores characterized by C2 symmetry and a relatively flexible structure resulting from its high Gly content. Peptide 1 has been synthesized by two different solid-phase protocols from its linear precursors, H-Gly-Gly-Lys(Fmoc)-Gly-Gly-Gly-Lys(Fmoc)-Gly-OH and H-Gly-Gly-Lys(Z)-Gly-Gly-Gly-Lys(Z)-Gly-OH), and satisfactorily characterized by chemical means. The CD spectrum of 1 is compatible with a beta-folded structure, stabilized by two internal hydrogen bonds. The complexation behavior of 1 toward alkaline and alkaline-earth cations can be envisaged as an equilibrium between inclusion (1:1) and sandwich (2:1) complex models, with affinities in the 10(6) M-1 and 10(11) M-2 range, respectively. A slight preference of 1 for Sr2+ over other cations has been found.
Ligand bindings of bovine carboxypeptidase B. III. Hydrophobic activators in dipeptide hydrolysis.[Pubmed:7400116]
J Biochem. 1980 Jun;87(6):1681-9.
Several hydrophobic compounds acted as activators in dipeptide (Bz-Gly-L-Arg-OH, Z-Gly-L-Phe-OH) hydrolysis by bovine carboxypeptidase B. These hydrophobic compounds include BZ-Gly-OH, Z-Gly-OH, Z-L-Phe-OH, and Z-L-Phe-GLy-OH. These compounds were indicated to bind to the secondary substrate binding sites which is proposed to be responsible for substrate activation kinetics in dipeptide hydrolysis. Of the compounds Z-L-Phe-OH alone acted also as a inhibitor at higher concentrations, indicating that it binds to both primary and secondary sites as the dipeptide substrates do. Comparison of the activation effects of the compounds employed indicated that hydrophobic interaction played an important role in binding to the secondary site. Substrate and modifier binding constants were also determined and the results indicated that modifier binding increased both affinity and catalytic rate constant of the primary site. On the other hand, Z-Gly-OH and Z-L-Phe-Gly-OH inhibited the hydrolyses of tri and tetrapeptide substrates. This observation suggests that the secondary site is contained in the extended active center which the enzyme possibly has.
The 1,3-dipolar cycloaddition reaction in the functionalization of carbon nanofibers.[Pubmed:18330154]
J Nanosci Nanotechnol. 2007 Oct;7(10):3441-5.
Carbon nanofibers were functionalized using a reaction scheme described in the literature for 1,3-dipolar cycloaddition of azomethine ylides generated in situ by the condensation of an alpha-amino acid and an aldehyde. The reagents used were Z-Gly-OH and paraformaldehyde. Their reaction with carbon nanofibers was studied as a solid mixture by controlled heating in the DSC. An oxazolidinone intermediate was formed as the major product. Z-Gly-OH and paraformaldehyde were also reacted with a model compound (anthracene) in DMF solution leading to the formation of a considerable amount of anthraquinone. These studies suggested that, under the conditions investigated, the 1,3-dipolar cycloaddition reaction was not favoured, and the main result of functionalization was the formation of quinone-type groups as a consequence of an oxidation process.


