YukovanolCAS# 76265-12-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 76265-12-8 | SDF | Download SDF |
| PubChem ID | 14542257 | Appearance | Powder |
| Formula | C20H18O6 | M.Wt | 354.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
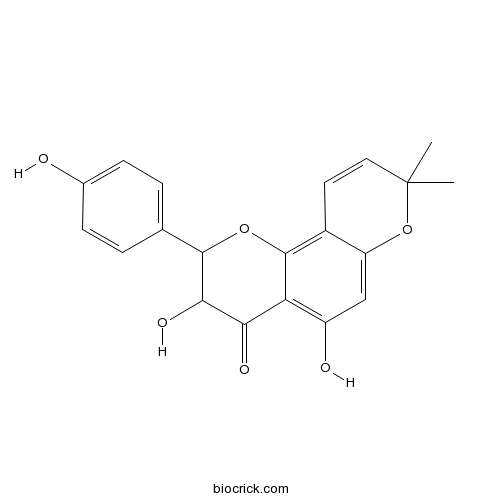
| Chemical Name | 3,5-dihydroxy-2-(4-hydroxyphenyl)-8,8-dimethyl-2,3-dihydropyrano[2,3-h]chromen-4-one | ||
| SMILES | CC1(C=CC2=C3C(=C(C=C2O1)O)C(=O)C(C(O3)C4=CC=C(C=C4)O)O)C | ||
| Standard InChIKey | CXIZZLWYTVCYIE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H18O6/c1-20(2)8-7-12-14(26-20)9-13(22)15-16(23)17(24)18(25-19(12)15)10-3-5-11(21)6-4-10/h3-9,17-18,21-22,24H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Yukovanol inhibits the proliferation of activated HSC-T6 cells in vitro. |

Yukovanol Dilution Calculator

Yukovanol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8209 mL | 14.1044 mL | 28.2087 mL | 56.4175 mL | 70.5219 mL |
| 5 mM | 0.5642 mL | 2.8209 mL | 5.6417 mL | 11.2835 mL | 14.1044 mL |
| 10 mM | 0.2821 mL | 1.4104 mL | 2.8209 mL | 5.6417 mL | 7.0522 mL |
| 50 mM | 0.0564 mL | 0.2821 mL | 0.5642 mL | 1.1283 mL | 1.4104 mL |
| 100 mM | 0.0282 mL | 0.141 mL | 0.2821 mL | 0.5642 mL | 0.7052 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Piptocarphin A
Catalog No.:BCN4586
CAS No.:76248-63-0
- 15-Hydroxyferruginol
Catalog No.:BCN3294
CAS No.:76235-93-3
- Ehretinine
Catalog No.:BCN1994
CAS No.:76231-29-3
- 5-Deoxycajanin
Catalog No.:BCN4310
CAS No.:7622-53-9
- Piptocarphin F
Catalog No.:BCN6447
CAS No.:76215-53-7
- Derrone
Catalog No.:BCN4587
CAS No.:76166-59-1
- TCPOBOP
Catalog No.:BCC6979
CAS No.:76150-91-9
- Mildronate
Catalog No.:BCC2289
CAS No.:76144-81-5
- TAE684 (NVP-TAE684)
Catalog No.:BCC3660
CAS No.:761439-42-3
- ALK inhibitor 2
Catalog No.:BCC1340
CAS No.:761438-38-4
- TAE226 (NVP-TAE226)
Catalog No.:BCC3885
CAS No.:761437-28-9
- ALK inhibitor 1
Catalog No.:BCC1339
CAS No.:761436-81-1
- Fmoc-Lys(Tfa)-OH
Catalog No.:BCC3524
CAS No.:76265-69-5
- Fmoc-Met(O)-OH
Catalog No.:BCC3530
CAS No.:76265-70-8
- 7-Deoxy-10-hydroxyloganetin
Catalog No.:BCN7578
CAS No.:76267-48-6
- Polyphyllin C
Catalog No.:BCN2587
CAS No.:76296-71-4
- Polyphyllin II
Catalog No.:BCN1052
CAS No.:76296-72-5
- Polyphyllin E
Catalog No.:BCN2588
CAS No.:76296-73-6
- Polyphyllin F
Catalog No.:BCN2589
CAS No.:76296-74-7
- Polyphyllin G
Catalog No.:BCN1054
CAS No.:76296-75-8
- Olaparib (AZD2281, Ku-0059436)
Catalog No.:BCC2206
CAS No.:763113-22-0
- Sodium Nitrite
Catalog No.:BCC4723
CAS No.:7632-00-0
- DL-AP5
Catalog No.:BCC6552
CAS No.:76326-31-3
- Latrunculin A
Catalog No.:BCC7830
CAS No.:76343-93-6
[Chemical constituents of Desmodium caudatum].[Pubmed:25090679]
Zhong Yao Cai. 2013 Dec;36(12):1953-6.
OBJECTIVE: To study the chemical constituents of Desmodium caudatum. METHODS: Silica column chromatography, Sephadex LH-20 column chromatography and recrystallization were used to separate and purify the chemical composition of Desmodium caudatum. Their chemical structures were identified by infrared spectrum (IR), mass spectrum (MS), nuclear magnetic resonance (NMR) and other physicochemical methods. RESULTS: Twelve compounds were isolated and identified as lacceroic acid(1), gheddic acid(2), stigmasterol(3), betulin(4), citrusinol(5), Yukovanol(6), kaempferol(7), protocatechuic acid(8), sophocarpine(9), matrine(10), N, Ndimethyltryptamine(11) and 5-hydroxy-N,N-dimethyltryptamine(12). CONCLUSION: Compounds 1, 2, 4 and 8-12 are isolated from this plant for the first time.
[Studies on constituents of rootsanel leaves from Desmodium blandum and their cytotoxic activity against growth of several tumor cells].[Pubmed:19160788]
Zhongguo Zhong Yao Za Zhi. 2008 Sep;33(18):2077-80.
OBJECTIVE: To investigate the chemical constituents of Desmodium blandum and their cytotoxic activity against the growth of several tumor cells. METHOD: Various chromatographic techniques including silica gel, Sephadex LH-20 column chromatography were employed for the isolation and purification of the constituents. The structures of compounds were elucidated by spectral analyses (IR, UV, NMR, MS). Their cytotoxic activity was then studied. RESULT: Eight compounds were isolated from the stems of D. blandum and identified as N, N-dimethyltryptamine (1), 5-methoxy-N, N-dimethyltryptamine (2), citrusinol (3), Yukovanol (4), (Z)-1-(4-hydroxy-2, 3-dimethoxyphenyl)-3-(4-hydroxyphenyl) propene (5), (Z)-1-(3-hydroxy-2, 4-dimethoxy-phenyl)-3-(4-hydroxy-3-methoxy-phenyl) propene (6), methylprotocatechuate (7), katuranin (8). CONCLUSION: Among these compounds, compound 6 was isolated from D. blandum for the first time. In the MTT test, compounds 2 and 6 exhibit cytotoxic activities against the KB cell, and compounds 3 and 6 exhibit the same activities against the HepG2 cell.
[Chemical constituents against hepatic fibrosis from Phyllodium pulchellum roots].[Pubmed:25174106]
Zhong Yao Cai. 2014 Mar;37(3):424-7.
OBJECTIVE: To investigate the bioactive constituents against hepatic fibrosis from the roots of Phyllodium pulchellum. METHODS: The chemical constituents of Phyllodium pulchellum roots were obtained by various chromatographic technologies and identified by several spectroscopic methods. RESULTS: Ten compounds were elucidated as 3,5,2',4'-tetrahydroxy-2",2"-dimethylpyrano-[5",6",7,8] -flavanone (1), Yukovanol (2), citflavanone (3), 8-prenylated 5,7,3',4'-tetrahydroxyflavanone (4), pulchelstyrene A (5), pulchelstyrene B (6), pulchelstyrene D (7), 3-indolcarbaldehyde (8), 3-methoxyindole (9) and p-hydroxybenzoic acid (10). The effects to inhibit the proliferation of activated HSC-T6 cells of all isolated compounds were also evaluated. CONCLUSION: All compounds are isolated from this plant for the first time except for compounds 5 - 7. Compounds 2,4,5 and 6 can inhibit the proliferation of activated HSC-T6 cells in vitro.


