XanthoxylinCAS# 90-24-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 90-24-4 | SDF | Download SDF |
| PubChem ID | 66654 | Appearance | Powder |
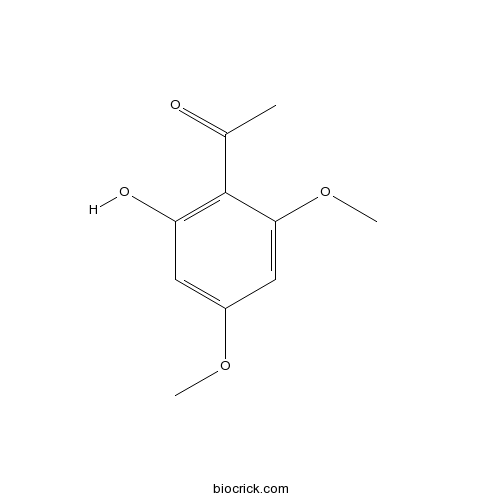
| Formula | C10H12O4 | M.Wt | 196.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(2-hydroxy-4,6-dimethoxyphenyl)ethanone | ||
| SMILES | CC(=O)C1=C(C=C(C=C1OC)OC)O | ||
| Standard InChIKey | FBUBVLUPUDBFME-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H12O4/c1-6(11)10-8(12)4-7(13-2)5-9(10)14-3/h4-5,12H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Xanthoxyline has antispasmodic, fungistatic, antinociceptive and antioedematogenic activities. Xanthoxylin has inhibitory effect on blood platelet aggregation; it induces melanogenesis mainly via cAMP-mediated PKA activation, other signaling pathways may also play a role in xanthoxylin-induced melanogenesis. |
| Targets | PKA | cAMP | PKC | Antifection |
| In vitro | Effect of xanthoxylin on melanin content and melanogenic protein expression in B16F10 melanoma[Reference: WebLink]Asian Biomed., 2012, 6(3):413-22.Reduced production of melanin and decreased or absence of melanocytes leads to various hypopigmentation disorders. Melanin synthesis is regulated by melanogenic proteins such as tyrosinase, tyrosinase-related protein 1 (TRP-1) and tyrosinase-related protein 2 (TRP -2), as well as their transcription factors. This study elucidated the effects of Xanthoxylin on melanin content, dendriticity, melanogenic protein expression and its signal transduction pathways in mouse B16F10 melanoma cells (B16F10 cells). Antispasmodic activity of xanthoxyline derivatives: structure-activity relationships.[Pubmed: 7629739]J Pharm Sci. 1995 Apr;84(4):473-5.
In vitro antifungal evaluation and studies on the mode of action of xanthoxyline derivatives.[Pubmed: 10635452]Arzneimittelforschung. 1999 Dec;49(12):1039-43.
|
| In vivo | Inhibitory Effect of Xanthoxylin on Blood Platelet Aggregation in Rabbits.[Reference: WebLink]Traditional Chinese Drug Research & Clinical Pharmacology,2000, 11(6):352-3.Turbidimetry was used to examine the inhibitory effect of Xanthoxyin on adenosine diphosphate (ADP)-, arachidic acid (AA )- and thrombin-induced platelet aggregation in rabbits. |
| Structure Identification | Zhong Yao Cai. 2014 Apr;37(4):608-10.Chemical constituents of n-BuOH extract from Phyllanthus matsumurae.[Pubmed: 25345134]To study the chemical constituents of n-BuOH extract from Phyllanthus matsumurae.
Eur. J. Med. Chem., 1996, 31(10):833-9.Synthesis of xanthoxyline derivatives with antinociceptive and antioedematogenic activities.[Reference: WebLink]Synthesis of Xanthoxyline Derivatives with Antinociceptive and Antioedematogenic Activities. |

Xanthoxylin Dilution Calculator

Xanthoxylin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0968 mL | 25.4842 mL | 50.9684 mL | 101.9368 mL | 127.421 mL |
| 5 mM | 1.0194 mL | 5.0968 mL | 10.1937 mL | 20.3874 mL | 25.4842 mL |
| 10 mM | 0.5097 mL | 2.5484 mL | 5.0968 mL | 10.1937 mL | 12.7421 mL |
| 50 mM | 0.1019 mL | 0.5097 mL | 1.0194 mL | 2.0387 mL | 2.5484 mL |
| 100 mM | 0.051 mL | 0.2548 mL | 0.5097 mL | 1.0194 mL | 1.2742 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- beta-Rhamnocitrin
Catalog No.:BCN3293
CAS No.:90-19-7
- 1-Naphthol
Catalog No.:BCC8473
CAS No.:90-15-3
- Guaiacol
Catalog No.:BCN8311
CAS No.:90-05-1
- Salicyl alcohol
Catalog No.:BCN4442
CAS No.:90-01-7
- 3-(Hydroxymethyl)-3-nitro-1-(4-octylphenyl)-1,4-butanediol
Catalog No.:BCN1313
CAS No.:899822-99-2
- 3-Nitro-1-(4-octylphenyl)-1-propanone
Catalog No.:BCN2250
CAS No.:899822-97-0
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
- ML 348
Catalog No.:BCC5611
CAS No.:899713-86-1
- A 438079
Catalog No.:BCC1316
CAS No.:899507-36-9
- Platycoside K
Catalog No.:BCN3242
CAS No.:899447-64-4
- A 438079 hydrochloride
Catalog No.:BCC1317
CAS No.:899431-18-6
- Gymnoside III
Catalog No.:BCN8218
CAS No.:899430-03-6
- 4-Methylumbelliferone
Catalog No.:BCN2563
CAS No.:90-33-5
- Xanthone
Catalog No.:BCC6493
CAS No.:90-47-1
- 3,4,5-Trimethoxycinnamic acid
Catalog No.:BCN5030
CAS No.:90-50-6
- 6-Amino-4-hydroxy-2-naphthalenesulfonic acid
Catalog No.:BCC8761
CAS No.:90-51-7
- Lobelin
Catalog No.:BCN2157
CAS No.:90-69-7
- 4,4'-Bis(diethylamino)benzophenone
Catalog No.:BCC8659
CAS No.:90-93-7
- Boc-Ser(PO3Bzl2)-OH
Catalog No.:BCC3443
CAS No.:90013-45-9
- PIK-293
Catalog No.:BCC4994
CAS No.:900185-01-5
- PIK-294
Catalog No.:BCC4995
CAS No.:900185-02-6
- Agar (bacteriological)
Catalog No.:BCC1208
CAS No.:9002-18-0
- PFI 4
Catalog No.:BCC6484
CAS No.:900305-37-5
- Detomidine HCl
Catalog No.:BCC4346
CAS No.:90038-01-0
[Chemical constituents of n-BuOH extract from Phyllanthus matsumurae].[Pubmed:25345134]
Zhong Yao Cai. 2014 Apr;37(4):608-10.
OBJECTIVE: To study the chemical constituents of n-BuOH extract from Phyllanthus matsumurae. METHODS: Column chromatography was used for the isolation and purification. Spectroscopic methods including H-NMR, 13C-NMR and MS were used for the identification of structures. RESULTS: Six compounds were isolated from the n-BuOH extract of 75% alcohol extract of the whole plant and identified as ellagic acid (1), phyllanthuspermin B (2), phyllanthuspermin C (3), Xanthoxylin (4), hesperetin-7-O-[6-O-(alpha-L-rhamnopy ranosyl)] -beta-D-glucopyranoside (5) and 4-O-methylgallic acid (6). CONCLUSION: Compounds 2 - 6 are obtained from this plant for the first time.
In vitro antifungal evaluation and studies on the mode of action of xanthoxyline derivatives.[Pubmed:10635452]
Arzneimittelforschung. 1999 Dec;49(12):1039-43.
This study describes the fungistatic effect of Xanthoxyline (CAS 90-24-4) and its derivatives against a panel of yeasts, filamentous fungi and dermatophytes, by using the agar dilution method. Results indicated that simple structural modifications led to more potent derivatives, especially in relation with dermatophytes. The most active compound tested (10), which is a benzenesulphonyl derivative, was 12-fold more potent than Xanthoxyline itself against Trichophyton rubrum. The evaluation of the mode of action with the whole cell Neurospora crassa assay, suggested that some selected compounds may be acting by the inhibition of fungal cell-wall polymers synthesis or assembly.
Antispasmodic activity of xanthoxyline derivatives: structure-activity relationships.[Pubmed:7629739]
J Pharm Sci. 1995 Apr;84(4):473-5.
The antispasmodic activity of several Xanthoxyline derivatives against acetylcholine-induced contraction of the guinea pig ileum was evaluated in vitro. The acetophenones with two methoxyl groups, mainly in the 3,4 positions, exhibited potent antispasmodic activity. Modification of the hydroxyl group in Xanthoxyline by the introduction of benzoyl, acetyl, or tosyl groups produced inactive compounds, whereas the introduction of benzyl or p-methoxybenzyl groups furnished compounds that were four- to eight-fold more potent than Xanthoxyline. In marked contrast, the introduction of a methyl group gave a compound that caused contractant activity. Modification of the carbonyl group of Xanthoxyline lead to inactive compounds, whereas the condensation of Xanthoxyline with benzaldehydes gave chalkones that were about fivefold more potent than Xanthoxyline. The introduction of benzyl and styrene groups, on the basis of the similarity with papaverine, improves the antispasmodic action of the Xanthoxyline derivates. Our results suggest that the methoxyl and carbonyl groups are critical structural points for the antispasmodic activity of Xanthoxyline derivatives. The hydroxyl group improves antispasmodic activity, but is not fundamental to its manifestation.


