ViolanthinCAS# 40581-17-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40581-17-7 | SDF | Download SDF |
| PubChem ID | 442665 | Appearance | Yellow powder |
| Formula | C27H30O14 | M.Wt | 578.52 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
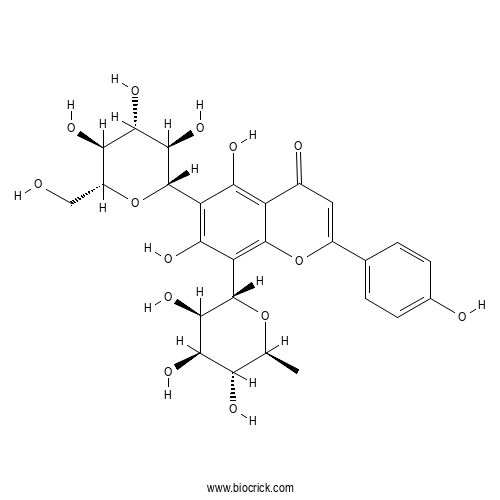
| Synonyms | Apigenin 6-C-glucoside 8-C-rhamnoside; 6-C-Glucosyl 8-C-rhamnosylapigenin; Isovitexin 8-C-rhamnoside; 8-C-Rhamnosylisovitexin; 4',5,7-Trihydroxyflavone 6-C-glucoside 8-C-rhamnoside | ||
| Solubility | Soluble in methan | ||
| Chemical Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-8-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]chromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)C2=C3C(=C(C(=C2O)C4C(C(C(C(O4)CO)O)O)O)O)C(=O)C=C(O3)C5=CC=C(C=C5)O)O)O)O | ||
| Standard InChIKey | MVOUGOXRXQDXDC-RSPRXDBDSA-N | ||
| Standard InChI | InChI=1S/C27H30O14/c1-8-17(31)21(35)23(37)27(39-8)16-20(34)15(26-24(38)22(36)18(32)13(7-28)41-26)19(33)14-11(30)6-12(40-25(14)16)9-2-4-10(29)5-3-9/h2-6,8,13,17-18,21-24,26-29,31-38H,7H2,1H3/t8-,13+,17-,18+,21+,22-,23+,24+,26-,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Violanthin Dilution Calculator

Violanthin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7285 mL | 8.6427 mL | 17.2855 mL | 34.571 mL | 43.2137 mL |
| 5 mM | 0.3457 mL | 1.7285 mL | 3.4571 mL | 6.9142 mL | 8.6427 mL |
| 10 mM | 0.1729 mL | 0.8643 mL | 1.7285 mL | 3.4571 mL | 4.3214 mL |
| 50 mM | 0.0346 mL | 0.1729 mL | 0.3457 mL | 0.6914 mL | 0.8643 mL |
| 100 mM | 0.0173 mL | 0.0864 mL | 0.1729 mL | 0.3457 mL | 0.4321 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isoviolanthin
Catalog No.:BCN0358
CAS No.:40788-84-9
- Junceine
Catalog No.:BCN0357
CAS No.:480-53-5
- Ruscogenin/Neoruscogenin mixture
Catalog No.:BCN0356
CAS No.:50933-59-0
- Trichodesmine N-oxide
Catalog No.:BCN0355
CAS No.:55727-46-3
- Sinapine chloride
Catalog No.:BCN0354
CAS No.:6484-80-6
- 7-Acetylintermedine N-oxide
Catalog No.:BCN0353
CAS No.:685132-59-6
- Echiumine N-oxide
Catalog No.:BCN0352
CAS No.:685554-68-1
- (+/-)-Praeruptorin B
Catalog No.:BCN0351
CAS No.:73069-26-8
- Tropine
Catalog No.:BCN0350
CAS No.:120-29-6
- α,β-Thujone
Catalog No.:BCN0349
CAS No.:76231-76-0
- Theacrine
Catalog No.:BCN0348
CAS No.:2309-49-1
- Tabersonine hydrochloride
Catalog No.:BCN0347
CAS No.:29479-00-3
- Glucolimnanthin potassium salt
Catalog No.:BCN0360
CAS No.:245550-59-8
- 11-Methylsulfinylundecylglucosinolate potassium salt
Catalog No.:BCN0361
CAS No.:186037-18-3
- 4'-Desulfocarboxyatractylic acid
Catalog No.:BCN0362
CAS No.:175447-19-5
- Dehydrocafestol
Catalog No.:BCN0363
CAS No.:155913-59-0
- Rinderine N-oxide
Catalog No.:BCN0364
CAS No.:137821-16-0
- Rebaudioside J
Catalog No.:BCN0365
CAS No.:1313049-59-0
- Condurango glycoside E2
Catalog No.:BCN0366
CAS No.:115784-09-3
- Dipsacus saponin A
Catalog No.:BCN0367
CAS No.:99624-66-5
- Dipsacus saponin B
Catalog No.:BCN0368
CAS No.:152406-42-3
- Dipsacus saponin C
Catalog No.:BCN0369
CAS No.:152406-43-4
- Acacetin 7-[rhamnosyl-(1->2)-galacturonide]
Catalog No.:BCN0370
CAS No.:38722-95-1
- Kaempferol 3,4'-di-O-glucoside
Catalog No.:BCN0371
CAS No.:71939-16-7
Molecular Docking Studies on the Anti-viral Effects of Compounds From Kabasura Kudineer on SARS-CoV-2 3CL(pro).[Pubmed:33425994]
Front Mol Biosci. 2020 Dec 23;7:613401.
The COVID-19 has now been declared a global pandemic by the World Health Organization. No approved drug is currently available; therefore, an urgent need has been developed for any antiviral therapy for COVID-19. Main protease 3CL(pro) of this novel Coronavirus (SARS-CoV-2) play a critical role in the disease propagation, and hence represent a crucial target for the drug discovery. Herein, we have applied a bioinformatics approach for drug repurposing to identify the possible potent inhibitors of SARS-CoV-2 main proteases 3CL(pro) (6LU7). In search of the anti-COVID-19 compound, we selected 145 phyto-compounds from Kabasura kudineer (KK), a poly-herbal formulation recommended by AYUSH for COVID-19 which are effective against fever, cough, sore throat, shortness of breath (similar to SARS-CoV2-like symptoms). The present study aims to identify molecules from natural products which may inhibit COVID-19 by acting on the main protease (3CL(pro)). Obtained results by molecular docking showed that Acetoside (-153.06), Luteolin 7 -rutinoside (-134.6) rutin (-133.06), Chebulagic acid (-124.3), Syrigaresinol (-120.03), Acanthoside (-122.21), Violanthin (-114.9), Andrographidine C (-101.8), myricetin (-99.96), Gingerenone -A (-93.9), Tinosporinone (-83.42), Geraniol (-62.87), Nootkatone (-62.4), Asarianin (-79.94), and Gamma sitosterol (-81.94) are main compounds from KK plants which may inhibit COVID-19 giving the better energy score compared to synthetic drugs. Based on the binding energy score, we suggest that these compounds can be tested against Coronavirus and used to develop effective antiviral drugs.
Identification of C-glycosyl flavones and quality assessment in Dendrobium nobile.[Pubmed:33238063]
Rapid Commun Mass Spectrom. 2021 Mar 30;35(6):e9012.
RATIONALE: Flavones are significant indicators of quality in traditional Chinese medicines (TCMs) and thus play a significant role in the quality control of TCMs in the pharmaceutical industry. Most flavones in Dendrobium nobile Lindl, a TCM with a long cultivation history and rich sources, have not been identified. This study was aimed at identifying the flavones in D. nobile from various habitats. METHODS: High-performance liquid chromatography (HPLC) coupled with diode-array detection and HPLC multiple-stage tandem mass spectrometry was used to identify the chemical constituents of D. nobile from various habitats, and a method was established to determine the content of vicenin II, Violanthin and isoViolanthin. Hierarchical cluster analysis, principal component analysis and orthogonal partial least-squares discriminant analysis were used to analyze the variations among 26 batches from different habitats. RESULTS: A total of 33 flavones were tentatively identified. Twenty-five flavones, previously undescribed in D. nobile, were acylated by p-coumaroyl, feruloyl, sinapoyl or 3-hydroxy-3-methylglutaryl. The D. nobile habitats were distinguished by significant differences in their flavone content. The C-glycosyl flavones were demonstrated to be characteristic compounds for evaluating D. nobile from various habitats. In particular, flavones acylated with 3-hydroxy-3-methylglutaryl were specific compounds that were only detected in samples from Yunnan. CONCLUSIONS: The results of this study could be used to improve the quality control of D. nobile and could provide references for the identification of acylated C-glycosyl flavones in other natural products.
Chemical Differentiation of Dendrobium officinale and Dendrobium devonianum by Using HPLC Fingerprints, HPLC-ESI-MS, and HPTLC Analyses.[Pubmed:28769988]
Evid Based Complement Alternat Med. 2017;2017:8647212.
The stems of Dendrobium officinale Kimura et Migo (Dendrobii Officinalis Caulis) have a high medicinal value as a traditional Chinese medicine (TCM). Because of the limited supply, D. officinale is a high priced TCM, and therefore adulterants are commonly found in the herbal market. The dried stems of a closely related Dendrobium species, Dendrobium devonianum Paxt., are commonly used as the substitute; however, there is no effective method to distinguish the two Dendrobium species. Here, a high performance liquid chromatography (HPLC) method was successfully developed and applied to differentiate D. officinale and D. devonianum by comparing the chromatograms according to the characteristic peaks. A HPLC coupled with electrospray ionization multistage mass spectrometry (HPLC-ESI-MS) method was further applied for structural elucidation of 15 flavonoids, 5 phenolic acids, and 1 lignan in D. officinale. Among these flavonoids, 4 flavonoid C-glycosides were firstly reported in D. officinale, and Violanthin and isoViolanthin were identified to be specific for D. officinale compared with D. devonianum. Then, two representative components were used as chemical markers. A rapid and reliable high performance thin layer chromatography (HPTLC) method was applied in distinguishing D. officinale from D. devonianum. The results of this work have demonstrated that these developed analytical methods can be used to discriminate D. officinale and D. devonianum effectively and conveniently.
[Separation and identification of specific components and quality standard of stem of Dendrobium officinale].[Pubmed:28905572]
Zhongguo Zhong Yao Za Zhi. 2016 Jul;41(13):2481-2486.
The Violanthin, a specific component, was separated and identified from the stems of Dendrobium officinale by chromatographic technique and spectroscopic method for the first time. The microscopic characteristics of D. officinale powder were examined under a microscopy and described. Thin layer chromatography (TLC) method was used for qualitative analysis of the Violanthin from D. officinale stems with a mixture of ethyl acetate, butanone, formic acid and water (4ratio3ratio1ratio1) as the developing solvent on high performance silica gel precoated plate (SGF254) and using aluminium trichloride as a chromagenic agent. The results showed significant characteristics of Violanthin from D. officinale stems on TLC, with certain specificity, and could be used to distinguish it from other easily confusing processed medicinal stems of D. devonianum, D. gratiosissimum and D. aphyllum. The content of naringenin, an active ingredient in D. officinale stems was determined by HPLC analysis on a Bischoff Chromatography HIPAK NC-04 ODS AB column (4.4 mmx250 mm, 5 mm) with acetonitrile-0.1% phosphoric acid solution as the mobile phase for gradient elution. The wavelength was set at 226 nm and column temperature was 25 . The HPLC method showed good linearity within the range of 3.90-250.00 g*mL(-)(1) (r = 0.999 9) for naringenin. The average recovery of naringenin was 99.20% with 0.17% of RSD. The mass fraction of 20 batches of D. officinale stems was between 0.190 and 0.498 mg*g(-)(1). The established qualitative and quantitative method was simple and rapid with good repeatability and accuracy, providing experimental basis for improving the quality standard of D. officinale, with a very important significance to ensure its quality and clinical effect.
Compounds from the aerial parts of Piper bavinum and their anti-cholinesterase activity.[Pubmed:25005067]
Arch Pharm Res. 2015;38(5):677-82.
A new alkenylphenol, bavinol A (1), together with six known compounds (2-7) were isolated from the aerial parts of Piper bavinum (Piperaceae). The chemical structures of these compounds were determined by spectroscopic analyses including 2D NMR spectroscopy. The anti-Alzheimer effects of compounds 1-7 were evaluated from acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity assays. Bavinol A (1), ampelopsin (3), and Violanthin (4) exhibited AChE inhibitory activities with IC50 values of 29.80, 59.47 and 79.80 muM. Compound 1 also showed the most potent BChE inhibitory activity with an IC50 value of 19.25 muM.
Quantitative and qualitative investigation of the main flavonoids in heartsease (Viola tricolor L.).[Pubmed:18366866]
J Chromatogr Sci. 2008 Feb;46(2):97-101.
Liquid chromatography coupled to electrospray ionization tandem mass spectrometry (MSn) is used for the analysis of flavonoids in heartsease (Viola tricolor L.). Our data suggested that the two main flavonoid components were Violanthin (6-C-glucosyl-8-C-rhamnosyl apigenin) and rutin (3-O-rutinosyl quercetin). The identification of rutin was confirmed by comparing its retention time, UV spectrum, molecular mass, and fragmentation pattern with the reference standard. In this paper, we also report on the quantitative analysis of rutin by high-performance liquid chromatography. According to our results, heartsease herb contained 420+/-1.17 microg/g rutin.
Major flavonoid components of heartsease (Viola tricolor L.) and their antioxidant activities.[Pubmed:18259733]
Anal Bioanal Chem. 2008 Apr;390(7):1917-25.
Sephadex LH-20 column chromatography was used to separate flavonoid components in a heartsease methanol extract. One of the main components was identified by NMR as Violanthin (6-C-glucosyl-8-C-rhamnosylapigenin). As a first approximation, the other main flavonoid component was considered to be rutin (3-O-rhamnoglucosylquercetin), based on comprehensive comparison of retention times and UV spectra of reference molecules, as well as molecular mass and fragmentation patterns obtained by mass spectrometry. The minor flavonoids were separated by polyamide column and analyzed by LC-MS. The antioxidant capacity of different flavonoid fractions was determined using both Trolox equivalent antioxidant capacity (TEAC) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) in vitro antioxidant assays. The highest electron-donor capacity was found for the major flavonoid component (rutin), whereas one minor component-rich flavonoid fraction exhibited the highest hydrogen-donor activity.
Flavonoids and other compounds from the aerial parts of Viola etrusca.[Pubmed:17311226]
Chem Biodivers. 2007 Feb;4(2):139-44.
The non-volatile constituents of the rare species Viola etrusca Erben (Violaceae), collected at Mount Amiata, Italy, were phytochemically investigated for the first time. Two new flavonoid glycosides, 4'-methoxyrhamnetin (1) and violetruscoside (2), an isorhamnetin derivative, were isolated from the flowering aerial parts, together with eleven known substances, including eight flavonoids, a phenolic acid, glycerin, and a coumarin derivative. The NMR data of Violanthin (10) and isoViolanthin (11) are reported for the first time.
[Principal constituents from flowering aerial parts of wild pansy].[Pubmed:11320336]
Ann Pharm Fr. 2001 Apr;59(2):119-24.
Dried flowering aerial parts of 12 harvested batches and 9 batches of commercial origin from Viola arvensis Murray were examined. The levels of principal compounds averaged respectively: total flavonoids 2.86 and 1.63%, rutin 1.15 and 0.57%, Violanthin 0.80 and 0.82%, violarvensin 0.75 and 0.20%, mucilage 21.5 and 16.5%, total ashes 10.6 and 14.8%, potassium 2.75 and 2.85% and also salicy lic acid 0.11 and 0.09%. Saponins were not detected. Specifications were discussed for an European Pharmacopoeial monography.
Violarvensin, a new flavone di-C-glycoside from Viola arvensis.[Pubmed:9548860]
J Nat Prod. 1998 Feb;61(2):272-4.
A new flavonoid di-C-glycoside, violarvensin (1), was isolated from the aerial parts of Viola arvensis, together with the known derivative Violanthin (2). The structure of 1 was established as apigenin-6-C-beta-D-glucopyranosyl-8-C-beta-D-6-deoxygulopyrano side by spectral analysis.
[HPLC analysis of flavonoids in the root of six Glycyrrhiza species].[Pubmed:2099092]
Yao Xue Xue Bao. 1990;25(11):840-8.
Ten flavonoids isolated from six species of Glycyrrhiza root were analysed by reversed phase HPLC. A column packed with Partisil 5 ODS-3 and gradient elution by using different percentage of methanol (B) in water-glacial acetic acid (97:3 V/V) (A) were used to separate licochalcone A, isoschaftoside, schaftoside, liquiritin, isoViolanthin, Violanthin, ononin, isoliquiritin, 4', 7-dihydroxyflavone and licoflavone A. Nine Glycyrrhiza samples of six species were analysed to determine their composition and content of the flavonoids. The results showed that the composition and content of the flavonoids of different Glycyrrhiza species were different as well as the same species but collected in different places.


