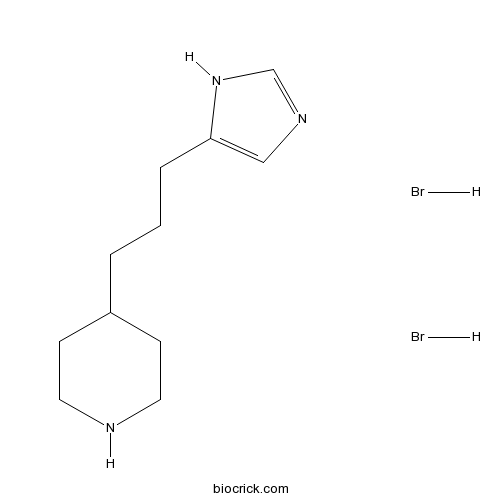
VUF 5681 dihydrobromidePotent H3 receptor silent antagonist CAS# 639089-06-8 |
- VU 0361737
Catalog No.:BCC4596
CAS No.:1161205-04-4
- Ifenprodil Tartrate
Catalog No.:BCC4589
CAS No.:23210-58-4
- VU 0364770
Catalog No.:BCC4597
CAS No.:61350-00-3
- MK-801 (Dizocilpine)
Catalog No.:BCC4591
CAS No.:77086-21-6
- Latrepirdine
Catalog No.:BCC4541
CAS No.:97657-92-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 639089-06-8 | SDF | Download SDF |
| PubChem ID | 53440140 | Appearance | Powder |
| Formula | C11H21Br2N3 | M.Wt | 355.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 4-[3-(1H-imidazol-5-yl)propyl]piperidine;dihydrobromide | ||
| SMILES | C1CNCCC1CCCC2=CN=CN2.Br.Br | ||
| Standard InChIKey | YFFVIGXPKWSZHW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H19N3.2BrH/c1(3-11-8-13-9-14-11)2-10-4-6-12-7-5-10;;/h8-10,12H,1-7H2,(H,13,14);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent histamine H3 receptor silent antagonist (pKi = 8.35). |

VUF 5681 dihydrobromide Dilution Calculator

VUF 5681 dihydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.816 mL | 14.0801 mL | 28.1603 mL | 56.3206 mL | 70.4007 mL |
| 5 mM | 0.5632 mL | 2.816 mL | 5.6321 mL | 11.2641 mL | 14.0801 mL |
| 10 mM | 0.2816 mL | 1.408 mL | 2.816 mL | 5.6321 mL | 7.0401 mL |
| 50 mM | 0.0563 mL | 0.2816 mL | 0.5632 mL | 1.1264 mL | 1.408 mL |
| 100 mM | 0.0282 mL | 0.1408 mL | 0.2816 mL | 0.5632 mL | 0.704 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- LH846
Catalog No.:BCC4246
CAS No.:639052-78-1
- Zinc Phytate
Catalog No.:BCN8302
CAS No.:63903-51-5
- Pinoresinol diglucoside
Catalog No.:BCN1093
CAS No.:63902-38-5
- Akuammidine
Catalog No.:BCN6509
CAS No.:639-36-1
- Ajmalidine
Catalog No.:BCN3491
CAS No.:639-30-5
- Gypsogenin
Catalog No.:BCC8993
CAS No.:639-14-5
- Cloxyfonac
Catalog No.:BCC5473
CAS No.:6386-63-6
- Meclofenamate Sodium
Catalog No.:BCC5490
CAS No.:6385-02-0
- NMDA (N-Methyl-D-aspartic acid)
Catalog No.:BCC4590
CAS No.:6384-92-5
- H-Glu-OMe
Catalog No.:BCC2924
CAS No.:6384-08-3
- Phyllostadimer A
Catalog No.:BCN4178
CAS No.:638203-32-4
- Z-D-Arg-OH
Catalog No.:BCC3575
CAS No.:6382-93-0
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- (3R,10S)-Heptadeca-1,8-diene-4,6-diyne-3,10-diol
Catalog No.:BCC9111
CAS No.:63910-76-9
- Artemisinin
Catalog No.:BCN5814
CAS No.:63968-64-9
- Hopeyhopin
Catalog No.:BCN7533
CAS No.:63975-56-4
- 13-Oxopodocarp-8(14)-en-18-oic acid
Catalog No.:BCN4009
CAS No.:63976-69-2
- Lobeline Hydrochloride
Catalog No.:BCC8202
CAS No.:63990-84-1
- Physostigmine hemisulfate
Catalog No.:BCC6724
CAS No.:64-47-1
- Demeclocycline hydrochloride
Catalog No.:BCC5303
CAS No.:64-73-3
- Tetracycline Hydrochloride
Catalog No.:BCC1206
CAS No.:64-75-5
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Etimizol
Catalog No.:BCC1562
CAS No.:64-99-3
Constitutive activity of H3 autoreceptors modulates histamine synthesis in rat brain through the cAMP/PKA pathway.[Pubmed:16769092]
Neuropharmacology. 2006 Sep;51(3):517-23.
We previously described that agonist-activated histamine H3 autoreceptors inhibit the stimulation of histamine synthesis mediated by calcium/calmodulin- and cAMP-dependent protein kinases (CaMKII and PKA respectively) in histaminergic nerve endings. In the absence of an agonist H3 receptors show partial constitutive activity, so we hypothesized that suppression of constitutive activity by an inverse agonist could stimulate these transduction pathways. We show here that the H3 inverse agonist thioperamide increases histamine synthesis in rat brain cortical slices independently from the amounts of extracellular histamine. Thioperamide effects were mimicked by the inverse agonists clobenpropit and A-331440, but not by the neutral antagonist VUF-5681. In contrast, coincubation with VUF-5681 suppressed thioperamide effects. The effects of thioperamide were completely blocked by the PKA inhibitor peptide myristoyl-PKI14-22, a peptide that did not block depolarization stimulation of histamine synthesis. In addition, thioperamide effects required depolarization and were impaired by blockade of N-type calcium channels (mediating depolarization), but not by CaMKII inhibition. These results indicate that constitutive activity of H3 receptors in rat brain cortex inhibits the adenylate cyclase/PKA pathway, and perhaps also the opening of N-type voltage sensitive calcium channels, but apparently not CaMKII.
The histamine H3 receptor: from gene cloning to H3 receptor drugs.[Pubmed:15665857]
Nat Rev Drug Discov. 2005 Feb;4(2):107-20.
Since the cloning of the histamine H(3) receptor cDNA in 1999 by Lovenberg and co-workers, this histamine receptor has gained the interest of many pharmaceutical companies as a potential drug target for the treatment of various important disorders, including obesity, attention-deficit hyperactivity disorder, Alzheimer's disease, schizophrenia, as well as for myocardial ischaemia, migraine and inflammatory diseases. Here, we discuss relevant information on this target protein and describe the development of various H(3) receptor agonists and antagonists, and their effects in preclinical animal models.
Identification of 4-(1H-imidazol-4(5)-ylmethyl)pyridine (immethridine) as a novel, potent, and highly selective histamine H(3) receptor agonist.[Pubmed:15115383]
J Med Chem. 2004 May 6;47(10):2414-7.
In this study, the piperidine ring of immepip and its analogues was replaced by a rigid heterocyclic pyridine ring. Many compounds in the series exhibit high affinity and agonist activity at the human histamine H(3) receptor. Particularly, the 4-pyridinyl analogue of immepip (1c, immethridine) is identified as a novel potent and highly selective histamine H(3) receptor agonist (pK(i) = 9.07, pEC(50) = 9.74) with a 300-fold selectivity over the closely related H(4) receptor.


