Tryprostatin ACAS# 171864-80-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 171864-80-5 | SDF | Download SDF |
| PubChem ID | 9929833 | Appearance | Powder |
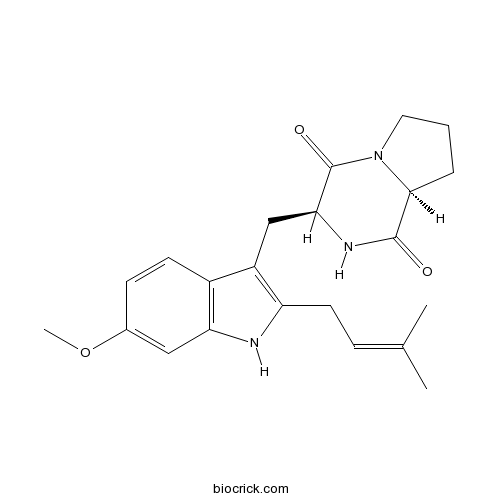
| Formula | C22H27N3O3 | M.Wt | 381.47 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,8aS)-3-[[6-methoxy-2-(3-methylbut-2-enyl)-1H-indol-3-yl]methyl]-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| SMILES | CC(=CCC1=C(C2=C(N1)C=C(C=C2)OC)CC3C(=O)N4CCCC4C(=O)N3)C | ||
| Standard InChIKey | XNRPVPHNDQHWLJ-PMACEKPBSA-N | ||
| Standard InChI | InChI=1S/C22H27N3O3/c1-13(2)6-9-17-16(15-8-7-14(28-3)11-18(15)23-17)12-19-22(27)25-10-4-5-20(25)21(26)24-19/h6-8,11,19-20,23H,4-5,9-10,12H2,1-3H3,(H,24,26)/t19-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Tryprostatin A and tryprostatin B are indole alkaloid-based fungal products that inhibit mammalian cell cycle at the G2/M phase. 2. Tryprostatin A is an inhibitor of breast cancer resistance protein. |
| Targets | Topoisomerase | Antifection |

Tryprostatin A Dilution Calculator

Tryprostatin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6214 mL | 13.1072 mL | 26.2144 mL | 52.4288 mL | 65.536 mL |
| 5 mM | 0.5243 mL | 2.6214 mL | 5.2429 mL | 10.4858 mL | 13.1072 mL |
| 10 mM | 0.2621 mL | 1.3107 mL | 2.6214 mL | 5.2429 mL | 6.5536 mL |
| 50 mM | 0.0524 mL | 0.2621 mL | 0.5243 mL | 1.0486 mL | 1.3107 mL |
| 100 mM | 0.0262 mL | 0.1311 mL | 0.2621 mL | 0.5243 mL | 0.6554 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-O-Coumaroylarjunolic acid
Catalog No.:BCN7131
CAS No.:171864-20-3
- Cauloside A
Catalog No.:BCN6726
CAS No.:17184-21-3
- 17-Acetyloxy-6-chloro-1α-chloromethylpregna-4,6-diene-3,20-dione
Catalog No.:BCC8440
CAS No.:17183-98-1
- Euchrenone A10
Catalog No.:BCN3574
CAS No.:171828-81-2
- 2-(1H-Indole-3-carboxamido)benzoic acid
Catalog No.:BCN1529
CAS No.:171817-95-1
- S-Adenosyl-L-Methtonine
Catalog No.:BCN2231
CAS No.:17176-17-9
- Compound 56
Catalog No.:BCC3615
CAS No.:171745-13-4
- Nitidanin
Catalog No.:BCN1107
CAS No.:171674-89-8
- Sodium Phenylbutyrate
Catalog No.:BCC2164
CAS No.:1716-12-7
- Sildenafil Citrate
Catalog No.:BCC2276
CAS No.:171599-83-0
- NBI-98854
Catalog No.:BCC4278
CAS No.:171598-74-6
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Palmatine chloride monohydrate
Catalog No.:BCC8228
CAS No.:171869-95-7
- SCH 51344
Catalog No.:BCC5613
CAS No.:171927-40-5
- H-Phe(4-NO2)-OMe.HCl
Catalog No.:BCC3294
CAS No.:17193-40-7
- Alpha-Tocotrienol
Catalog No.:BCN3724
CAS No.:1721-51-3
- Gericudranin E
Catalog No.:BCN8070
CAS No.:172104-07-3
- Ilaprazole
Catalog No.:BCC9000
CAS No.:172152-36-2
- Mepivacaine HCl
Catalog No.:BCC4796
CAS No.:1722-62-9
- PKC β pseudosubstrate
Catalog No.:BCC5811
CAS No.:172308-76-8
- Scabioside C
Catalog No.:BCC8358
CAS No.:17233-22-6
- 2-Hydroxy-3-methoxybenzoic acid glucose ester
Catalog No.:BCN7407
CAS No.:172377-87-6
- Dihydrodaidzein
Catalog No.:BCN2819
CAS No.:17238-05-0
- 2-Hydroxy-3-methylanthraquinone
Catalog No.:BCN1108
CAS No.:17241-40-6
Reversal of breast cancer resistance protein-mediated drug resistance by tryprostatin A.[Pubmed:14566821]
Int J Cancer. 2003 Dec 10;107(5):721-8.
MDR in human cancers is one of the major causes of failure of chemotherapy. A member of the superfamily of ABC transporters, BCRP, was demonstrated to confer an atypical MDR phenotype to tumor cells. To overcome the BCRP-mediated drug resistance, the fungal secondary metabolite TPS-A, a diketopiperazine, was analyzed with regard to its potency to reverse the BCRP-mediated drug-resistant phenotype. At concentrations of 10-50 microM, TPS-A reversed a mitoxantrone-resistant phenotype and inhibited the cellular BCRP-dependent mitoxantrone accumulation in the human gastric carcinoma cell line EPG85-257RNOV, the human breast cancer cell line MCF7/AdrVp (both exhibiting acquired BCRP-mediated MDR) and the BCRP cDNA-transfected breast cancer cell line MCF-7/BCRP clone 8. No cytotoxicity was seen at effective concentrations. These data indicate that TPS-A is a novel BCRP inhibitor.
Synthesis and structure-activity relationship studies on tryprostatin A, an inhibitor of breast cancer resistance protein.[Pubmed:18321710]
Bioorg Med Chem. 2008 Apr 15;16(8):4626-51.
Tryprostatin A is an inhibitor of breast cancer resistance protein, consequently a series of structure-activity studies on the cell cycle inhibitory effects of Tryprostatin A analogues as potential antitumor antimitotic agents have been carried out. These analogues were assayed for their growth inhibition properties and their ability to perturb the cell cycle in tsFT210 cells. SAR studies resulted in the identification of the essential structural features required for cytotoxic activity. The absolute configuration L-Tyr-L-pro in the diketopiperazine ring along with the presence of the 6-methoxy substituent on the indole moiety of 1 was shown to be essential for dual inhibition of topoisomerase II and tubulin polymerization. Biological evaluation also indicated the presence of the 2-isoprenyl moiety on the indole scaffold of 1 was essential for potent inhibition of cell proliferation. Substitution of the indole N(a)-H in 1 with various alkyl or aryl groups, incorporation of various L-amino acids into the diketopiperazine ring in place of L-proline, and substitution of the 6-methoxy group in 1 with other functionality provided active analogues. The nature of the substituents present on the indole N(a)-H or the indole C-2 position influenced the mechanism of action of these analogues. Analogues 68 (IC(50)=10 microM) and 67 (IC(50)=19 microM) were 7-fold and 3.5-fold more potent, respectively, than 1 (IC(50)=68 microM) in the inhibition of the growth of tsFT210 cells. Diastereomer-2 of tryprostatin B 8 was a potent inhibitor of the growth of three human carcinoma cell lines: H520 (IC(50)=11.9 microM), MCF-7 (IC(50)=17.0 microM) and PC-3 (IC(50)=11.1 microM) and was equipotent with etoposide, a clinically used anticancer agent. Isothiocyanate analogue 71 and 6-azido analogue 72 were as potent as 1 in the tsFT210 cell proliferation and may be useful tools in labeling BCRP.
Engineered Production of Tryprostatins in E. coli through Reconstitution of a Partial ftm Biosynthetic Gene Cluster from Aspergillus sp.[Pubmed:26640821]
Jacobs J Biotechnol Bioeng. 2015 Apr;2(1). Epub 2014 Dec 18.
Tryprostatin A and B are indole alkaloid-based fungal products that inhibit mammalian cell cycle at the G2/M phase. They are biosynthetic intermediates of fumitremorgins produced by a complex pathway involving a nonribosomal peptide synthetase (FtmA), a prenyltransferase (FtmB), a cytochrome P450 hydroxylase (FtmC), an O-methyltransferase (FtmD), and several additional enzymes. A partial fumitremorgin biosynthetic gene cluster (ftmABCD) from Aspergillus sp. was reconstituted in Escherichia coli BL21(DE3) cells, with or without the co-expression of an Sfp-type phosphopantetheinyltransferase gene (Cv_sfp) from Chromobacterium violaceum No. 968. Several recombinant E. coli strains produced tryprostatin B up to 106 mg/l or Tryprostatin A up to 76 mg/l in the fermentation broth under aerobic condition, providing an effective way to prepare those pharmaceutically important natural products biologically.


