Tiagabine hydrochlorideGABA uptake inhibitor; anticonvulsant CAS# 145821-59-6 |
- BS-181 HCl
Catalog No.:BCC2537
CAS No.:1397219-81-6
- PHA-793887
Catalog No.:BCC2521
CAS No.:718630-59-2
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 145821-59-6 | SDF | Download SDF |
| PubChem ID | 91274 | Appearance | Powder |
| Formula | C20H26ClNO2S2 | M.Wt | 412.01 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NO050328 hydrochloride; NO328 hydrochloride; TGB hydrochloride | ||
| Solubility | DMSO : ≥ 53 mg/mL (128.64 mM) *"≥" means soluble, but saturation unknown. | ||
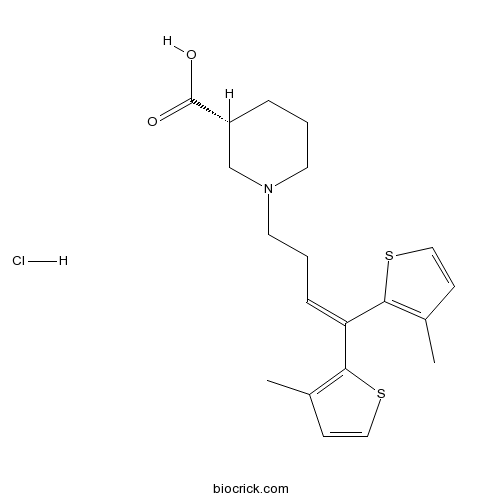
| Chemical Name | (3R)-1-[4,4-bis(3-methylthiophen-2-yl)but-3-enyl]piperidine-3-carboxylic acid;hydrochloride | ||
| SMILES | CC1=C(SC=C1)C(=CCCN2CCCC(C2)C(=O)O)C3=C(C=CS3)C.Cl | ||
| Standard InChIKey | YUKARLAABCGMCN-PKLMIRHRSA-N | ||
| Standard InChI | InChI=1S/C20H25NO2S2.ClH/c1-14-7-11-24-18(14)17(19-15(2)8-12-25-19)6-4-10-21-9-3-5-16(13-21)20(22)23;/h6-8,11-12,16H,3-5,9-10,13H2,1-2H3,(H,22,23);1H/t16-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GABA uptake inhibitor (IC50 = 67 nM in vivo). Exhibits high affinity and selectivity for the GAT-1 GABA transporter. Anticonvulsant; also attenuates established dynorphin-induced allodynia in a mouse model after systemic administration. |

Tiagabine hydrochloride Dilution Calculator

Tiagabine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4271 mL | 12.1356 mL | 24.2713 mL | 48.5425 mL | 60.6781 mL |
| 5 mM | 0.4854 mL | 2.4271 mL | 4.8543 mL | 9.7085 mL | 12.1356 mL |
| 10 mM | 0.2427 mL | 1.2136 mL | 2.4271 mL | 4.8543 mL | 6.0678 mL |
| 50 mM | 0.0485 mL | 0.2427 mL | 0.4854 mL | 0.9709 mL | 1.2136 mL |
| 100 mM | 0.0243 mL | 0.1214 mL | 0.2427 mL | 0.4854 mL | 0.6068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tiagabine hydrochloride(NO328 hydrochloride) is a selective gamma-aminobutyric acid (GABA) reuptake inhibitor. Target: GABA reuptake inhibitor Tiagabine had an early onset of effect, as shown by significant reduction from baseline in mean HAM-A total score compared with placebo at week 1 (observed cases, p < .05). Tiagabine was generally well tolerated and not associated with changes in sexual functioning or depressive status. Symptoms of a discontinuation syndrome during taper were not observed. Tiagabine may be a useful treatment option for adult patients diagnosed with GAD [1]. Tiagabine was generally well tolerated; the most common adverse events were nausea, dizziness and headaches [2]. Tiagabine (0.1 microM), an antiepileptic drug that specifically inhibits the GAT-1 GABA transporter inhibited GABA uptake 50% in temporal cortex and 60-68% in white structures [3].
References:
[1]. Pollack, M.H., et al., The selective GABA reuptake inhibitor tiagabine for the treatment of generalized anxiety disorder: results of a placebo-controlled study. J Clin Psychiatry, 2005. 66(11): p. 1401-8.
[2]. Sheehan, D.V., et al., An open-label study of tiagabine in panic disorder. Psychopharmacol Bull, 2007. 40(3): p. 32-40.
[3]. Henjum, S. and B. Hassel, High-affinity GABA uptake and GABA-metabolizing enzymes in pig forebrain white matter: a quantitative study. Neurochem Int, 2007. 50(2): p. 365-70.
- Margatoxin
Catalog No.:BCC7709
CAS No.:145808-47-5
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine
Catalog No.:BCC8666
CAS No.:145783-15-9
- 4,6-Dichloro-5-nitro-2-propylthiopyrimidine
Catalog No.:BCC8667
CAS No.:145783-14-8
- AR-C 66096 tetrasodium salt
Catalog No.:BCC6004
CAS No.:145782-74-7
- 3-Allylrhodanine
Catalog No.:BCC8604
CAS No.:1457-47-2
- HG-9-91-01
Catalog No.:BCC4071
CAS No.:1456858-58-4
- SH-4-54
Catalog No.:BCC5483
CAS No.:1456632-40-8
- 2'-O-Benzoylpaeoniflorin
Catalog No.:BCN7803
CAS No.:1456598-64-3
- NNC 711
Catalog No.:BCC7176
CAS No.:145645-62-1
- Cyclocommunol
Catalog No.:BCN3375
CAS No.:145643-96-5
- Sophocarpine
Catalog No.:BCN5971
CAS No.:145572-44-7
- D-myo-Inositol-1,3,4,5-tetrakisphosphate, octapotassium salt
Catalog No.:BCC7058
CAS No.:145843-69-2
- Brachynoside
Catalog No.:BCN3749
CAS No.:145898-87-9
- CGP 52411
Catalog No.:BCC7667
CAS No.:145915-58-8
- CGP 53353
Catalog No.:BCC7363
CAS No.:145915-60-2
- 7,8,9,9-Tetradehydroisolariciresinol
Catalog No.:BCN1649
CAS No.:145918-59-8
- 3-Amino-3-phenyl-1-propanol
Catalog No.:BCC8608
CAS No.:14593-04-5
- 2-(Methylamino)ethylphosphonic acid
Catalog No.:BCN1763
CAS No.:14596-55-5
- 2-Dimethylaminoethylphosphonic acid
Catalog No.:BCN1764
CAS No.:14596-56-6
- N,N,N-Trimethyl-2-aminoethylphosphonate
Catalog No.:BCN1560
CAS No.:14596-57-7
- Laccaic acid E
Catalog No.:BCN1807
CAS No.:14597-16-1
- Jasminoid A
Catalog No.:BCN7605
CAS No.:1459784-57-6
- Cycloart-23-ene-3,25-diol
Catalog No.:BCN2640
CAS No.:14599-48-5
Comparison of antiepileptic drugs tiagabine, lamotrigine, and gabapentin in mouse models of acute, prolonged, and chronic nociception.[Pubmed:12183677]
J Pharmacol Exp Ther. 2002 Sep;302(3):1168-75.
Some antiepileptic drugs have been shown to be clinically effective in the treatment of neuropathic pain. This study determined whether the new antiepileptic drug tiagabine, a GABA uptake inhibitor, is efficacious in mice in a broad range of nociceptive tests (hot-plate, formalin, and dynorphin-induced chronic allodynia) and compared tiagabine's potency with two other antiepileptic drugs, gabapentin and lamotrigine. Intraperitoneally administered tiagabine, but not lamotrigine, gabapentin, or i.t. tiagabine, produced dose-dependent antinoception in the hot-plate test. A 5-min pretreatment with tiagabine (2-29 nmol i.t.) dose-dependently inhibited both the acute and late phase formalin behaviors; pretreatment with lamotrigine (4-265 nmol i.t.) inhibited only the late phase. In the formalin assay the GABA(A) antagonist bicuculline reversed the acute phase antinociception, whereas the GABA(B) antagonist saclofen reversed both the acute and late phase tiagabine-induced antinociception. Tiagabine administered i.p. but not i.t. dose-dependently reduced dynorphin-induced chronic allodynia for 120 min. Gabapentin and lamotrigine produced antinociception administered either i.t. or i.p. in a dose-dependent manner. Thus, we have shown that gabapentin and lamotrigine produced antinociception in two mouse models of pain, whereas tiagabine produced antinociception in all three mouse models of pain.
Design, synthesis and evaluation of substituted triarylnipecotic acid derivatives as GABA uptake inhibitors: identification of a ligand with moderate affinity and selectivity for the cloned human GABA transporter GAT-3.[Pubmed:8057281]
J Med Chem. 1994 Jul 22;37(15):2334-42.
gamma-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian central nervous system. Molecular biology has revealed the presence of four high-affinity GABA transporters in the brain, GAT-1, GAT-2, GAT-3, and BGT-1, the latter transporting both GABA and the osmolyte Betaine. We have shown that known GABA uptake inhibitors such as SK&F 89976-A, CI-966, and Tiagabine exhibit high affinity and selectivity for GAT-1. In the present paper we describe the design and synthesis of a novel series of triarylnipecotic acid derivatives for evaluation as GABA uptake inhibitors. The design lead for this series of compounds was the nonselective GABA uptake inhibitor EGYT-3886, [(-)-2-phenyl-2-[(dimethylamino)ethoxy]-(1R)- 1,7,7-trimethylbicyclo[2.2.1]heptane]. From this series of compounds (S)-1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-3-piperidinecarboxylic+ ++ (S)-1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-3-piperidinecarboxylic+ ++ acid, 4(S) was identified as a novel ligand with selectivity for GAT-3. 4(S) displayed an IC50 of 5 microM at GAT-3, 21 microM at GAT-2, > 200 microM at GAT-1, and 140 microM at BGT-1. This compound will be an important tool for evaluating the role of GAT-3 in neural function.
The synthesis of novel GABA uptake inhibitors. 1. Elucidation of the structure-activity studies leading to the choice of (R)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]-3-piperidinecarboxylic acid (tiagabine) as an anticonvulsant drug candidate.[Pubmed:8510100]
J Med Chem. 1993 Jun 11;36(12):1716-25.
A series of different synthetic approaches to novel GABA uptake inhibitors are described, leading to examples which are derivatives of nipecotic acid and guvacine, substituted at nitrogen by 4,4-diaryl-3-butenyl or 2-(diphenylmethoxy)ethyl moieties. The in vitro value for inhibition of [3H]-GABA uptake in rat synaptosomes was determined for each compound. It was found that the most potent examples are those having a substituent in an "ortho" position in one or both aromatic/heteroaromatic groups. The majority of the compounds described are structurally related to tiagabine, (R)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]-3- piperidinecarboxylic acid hydrochloride (NNC 05-0328) and some of the reasoning behind the selection of this compound as a drug candidate is summarized.


