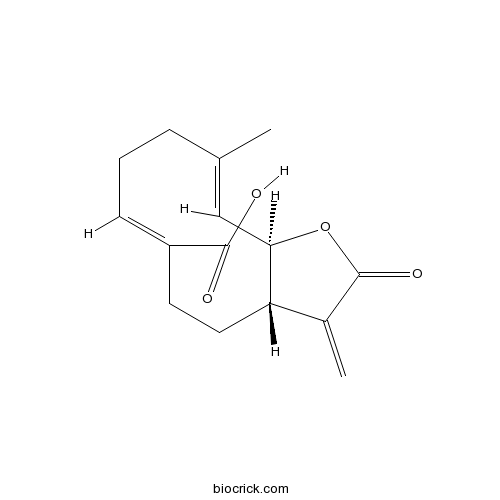
Taraxinic acidCAS# 75911-33-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75911-33-0 | SDF | Download SDF |
| PubChem ID | 9921439 | Appearance | Powder |
| Formula | C15H18O4 | M.Wt | 262.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3aS,6Z,10E,11aR)-10-methyl-3-methylidene-2-oxo-3a,4,5,8,9,11a-hexahydrocyclodeca[b]furan-6-carboxylic acid | ||
| SMILES | CC1=CC2C(CCC(=CCC1)C(=O)O)C(=C)C(=O)O2 | ||
| Standard InChIKey | KZWCOWFKXMTBRH-ZWXOUNMOSA-N | ||
| Standard InChI | InChI=1S/C15H18O4/c1-9-4-3-5-11(14(16)17)6-7-12-10(2)15(18)19-13(12)8-9/h5,8,12-13H,2-4,6-7H2,1H3,(H,16,17)/b9-8+,11-5-/t12-,13+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Taraxinic acid Dilution Calculator

Taraxinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8124 mL | 19.0621 mL | 38.1243 mL | 76.2486 mL | 95.3107 mL |
| 5 mM | 0.7625 mL | 3.8124 mL | 7.6249 mL | 15.2497 mL | 19.0621 mL |
| 10 mM | 0.3812 mL | 1.9062 mL | 3.8124 mL | 7.6249 mL | 9.5311 mL |
| 50 mM | 0.0762 mL | 0.3812 mL | 0.7625 mL | 1.525 mL | 1.9062 mL |
| 100 mM | 0.0381 mL | 0.1906 mL | 0.3812 mL | 0.7625 mL | 0.9531 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,7-Bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one
Catalog No.:BCN9572
CAS No.:149732-52-5
- Isohopeaphenol
Catalog No.:BCN9571
CAS No.:197446-77-8
- Hopeaphenol
Catalog No.:BCN9570
CAS No.:388582-37-4
- Myricetin 3,7,3'-trimethyl ether 5'-O-glucoside
Catalog No.:BCN9569
CAS No.:2170444-56-9
- 3,4-Dihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9568
CAS No.:454473-97-3
- 3,4,5-Trihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9567
CAS No.:2172431-63-7
- Pukateine
Catalog No.:BCN9566
CAS No.:81-67-4
- 11-Methylforsythide
Catalog No.:BCN9565
CAS No.:159598-00-2
- Adoxoside
Catalog No.:BCN9564
CAS No.:42830-26-2
- 3-O-Methylellagic acid 4-O-rhamnoside
Catalog No.:BCN9563
CAS No.:639089-97-7
- Quercetin 3,5,3'-trimethyl ether
Catalog No.:BCN9562
CAS No.:13459-09-1
- Aesculioside C
Catalog No.:BCN9561
CAS No.:254896-65-6
- Harringtonolide
Catalog No.:BCN9574
CAS No.:64761-48-4
- Caryatin
Catalog No.:BCN9575
CAS No.:1486-66-4
- Demethylagrimonolide 6-O-glucoside
Catalog No.:BCN9576
CAS No.:1257408-55-1
- Momordicin IV
Catalog No.:BCN9577
CAS No.:894412-35-2
- Taikuguasin D
Catalog No.:BCN9578
CAS No.:1627163-80-7
- Karaviloside XI
Catalog No.:BCN9579
CAS No.:934739-35-2
- Hainanolidol
Catalog No.:BCN9580
CAS No.:73213-63-5
- Fortunolide A
Catalog No.:BCN9581
CAS No.:252574-51-9
- Actinodaphnine
Catalog No.:BCN9582
CAS No.:517-69-1
- Goyaglycoside d
Catalog No.:BCN9583
CAS No.:333332-50-6
- 3'-Hydroxy-3,5,6,7,8,4',5'-heptamethoxyflavone
Catalog No.:BCN9584
CAS No.:5244-28-0
- (+)-Isoampelopsin F
Catalog No.:BCN9585
CAS No.:354553-38-1
A new sesquiterpenoid and further natural products from Taraxacum portentosum Kirschner & Stepanek, an endangered species.[Pubmed:31928353]
Nat Prod Res. 2020 Jan 13:1-5.
The chemical studies of roots and aerial parts of Taraxacum portentosum Kirschner & Stepanek, a member of the section Palustria (H. Lindb.) Dahlst. (Asteraceae), led to the isolation of one new eudesmanolide and 13 known compounds, including five sesquiterpenoids: Taraxinic acid, 11beta,13-dihydroTaraxinic acid, Taraxinic acid beta-glucopyranosyl ester and its 11beta,13-dihydroderivative, ixerin D, one apocarotenoid loliolide and seven phenolics: scopoletin, 3-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-1-propanone, methyl p-hydroxyphenylacetate, 5-methoxy-eugenyl-4-O-beta-glucopyranoside, syringin, dihydroconiferin, and dihydrosyringin. Their structures were established by (1)H NMR. The new compound was characterized as 3-oxo-4betaH-11,13-eudesmen-12,6-olide-8-O-beta-glucopyranoside based on spectroscopic data (1 D and 2 D NMR) and HRESI mass spectrometry.
Impact of Seasonal and Temperature-Dependent Variation in Root Defense Metabolites on Herbivore Preference in Taraxacum officinale.[Pubmed:31832894]
J Chem Ecol. 2020 Jan;46(1):63-75.
Plants experience seasonal fluctuations in abiotic and biotic factors such as herbivore attack rates. If and how root defense expression co-varies with seasonal fluctuations in abiotic factors and root herbivore attack rates is not well understood. Here, we evaluated seasonal changes in defensive root latex chemistry of Taraxacum officinale plants in the field and correlated the changes with seasonal fluctuations in abiotic factors and damage potential by Melolontha melolontha, a major natural enemy of T. officinale. We then explored the causality and consequences of these relationships under controlled conditions. The concentration of the defensive sesquiterpene lactone Taraxinic acid beta-D glucopyranosyl ester (TA-G) varied substantially over the year and was most strongly correlated to mean monthly temperature. Both temperature and TA-G levels were correlated with annual fluctuations in potential M. melolontha damage. Under controlled conditions, plants grown under high temperature produced more TA-G and were less attractive for M. melolontha. However, temperature-dependent M. melolontha feeding preferences were not significantly altered in TA-G deficient transgenic lines. Our results suggest that fluctuations in temperature leads to variation in the production of a root defensive metabolites that co-varies with expected attack of a major root herbivore. Temperature-dependent herbivore preference, however, is likely to be modulated by other phenotypic alterations.
Ethnobotany of the genus Taraxacum-Phytochemicals and antimicrobial activity.[Pubmed:30039597]
Phytother Res. 2018 Nov;32(11):2131-2145.
Plants belonging to the genus Taraxacum have been used in traditional healthcare to treat infectious diseases including food-borne infections. This review aims to summarize the available information on Taraxacum spp., focusing on plant cultivation, ethnomedicinal uses, bioactive phytochemicals, and antimicrobial properties. Phytochemicals present in Taraxacum spp. include sesquiterpene lactones, such as taraxacin, mongolicumin B, and Taraxinic acid derivatives; triterpenoids, such as taraxasterol, taraxerol, and officinatrione; and phenolic derivatives, such as hydroxycinnamic acids (chlorogenic, chicoric, and caffeoyltartaric acids), coumarins (aesculin and cichoriin), lignans (mongolicumin A), and taraxacosides. Aqueous and organic extracts of different plant parts exhibit promising in vitro antimicrobial activity relevant for controlling fungi and Gram-positive and Gram-negative bacteria. Therefore, this genus represents a potential source of bioactive phytochemicals with broad-spectrum antimicrobial activity. However, so far, preclinical evidence for these activities has not been fully substantiated by clinical studies. Indeed, clinical evidence for the activity of Taraxacum bioactive compounds is still scant, at least for infectious diseases, and there is limited information on oral bioavailability, pharmacological activities, and safety of Taraxacum products in humans, though their traditional uses would suggest that these plants are safe.
[Chemical constituents of Myripnois dioica].[Pubmed:28920380]
Zhongguo Zhong Yao Za Zhi. 2016 Sep;41(17):3260-3264.
To study the chemical constituents of the aerial parts of Myripnois dioica. Twelve compounds were separated from the 95% ethanol extract of M. dioica by using various chromatographic techniques. Their stuctures were identified on the basis of their physicochemical properties and spectral data as 8-desoxyurospermal A(1), zaluzanin C(2), dehydrozaluzanin C(3), glucozaluzanin C(4), macrocliniside B(5), macrocliniside I(6), Taraxinic acid-14-O-beta-D-glucopyranoside(7), ainsliaside B(8), apigenin(9), luteolin(10), apigenin-7-O-beta-D-glucopyranoside(11), and luteolin-7-O-beta-D-glucopyranoside(12). Except for compound 8, the other compounds were isolated from this genus for the first time. Compound 8 was found to decrease blood glucose level properly in alloxan-induced diabetic mice.
A Herbivore Tag-and-Trace System Reveals Contact- and Density-Dependent Repellence of a Root Toxin.[Pubmed:28303526]
J Chem Ecol. 2017 Mar;43(3):295-306.
Foraging behavior of root feeding organisms strongly affects plant-environment-interactions and ecosystem processes. However, the impact of plant chemistry on root herbivore movement in the soil is poorly understood. Here, we apply a simple technique to trace the movement of soil-dwelling insects in their habitats without disturbing or restricting their interactions with host plants. We tagged the root feeding larvae of Melolontha melolontha with a copper ring and repeatedly located their position in relation to their preferred host plant, Taraxacum officinale, using a commercial metal detector. This method was validated and used to study the influence of the sesquiterpene lactone Taraxinic acid beta-D-glucopyranosyl ester (TA-G) on the foraging of M. melolontha. TA-G is stored in the latex of T. officinale and protects the roots from herbivory. Using behavioral arenas with TA-G deficient and control plants, we tested the impact of physical root access and plant distance on the effect of TA-G on M. melolontha. The larvae preferred TA-G deficient plants to control plants, but only when physical root contact was possible and the plants were separated by 5 cm. Melolontha melolontha showed no preference for TA-G deficient plants when the plants were grown 15 cm apart, which may indicate a trade-off between the cost of movement and the benefit of consuming less toxic food. We demonstrate that M. melolontha integrates host plant quality and distance into its foraging patterns and suggest that plant chemistry affects root herbivore behavior in a plant-density dependent manner.
Sesquiterpene Lactone Composition and Cellular Nrf2 Induction of Taraxacum officinale Leaves and Roots and Taraxinic Acid beta-d-Glucopyranosyl Ester.[Pubmed:28026992]
J Med Food. 2017 Jan;20(1):71-78.
Taraxacum officinale, the common dandelion, is a plant of the Asteraceae family, which is used as a food and medical herb. Various secondary plant metabolites such as sesquiterpene lactones, triterpenoids, flavonoids, phenolic acids, coumarins, and steroids have been described to be present in T. officinale. Dandelion may exhibit various health benefits, including antioxidant, anti-inflammatory, and anticarcinogenic properties. We analyzed the leaves and roots of the common dandelion (T. officinale) using high-performance liquid chromatography/mass spectrometry to determine its sesquiterpene lactone composition. The main compound of the leaf extract Taraxinic acid beta-d-glucopyranosyl ester (1), a sesquiterpene lactone, was isolated and the structure elucidation was conducted by nuclear magnetic resonance spectrometry. The leaf extract and its main compound 1 activated the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) in human hepatocytes more significantly than the root extract. Furthermore, the leaf extract induced the Nrf2 target gene heme oxygenase 1. Overall, present data suggest that compound 1 may be one of the active principles of T. officinale.
A below-ground herbivore shapes root defensive chemistry in natural plant populations.[Pubmed:27009228]
Proc Biol Sci. 2016 Mar 30;283(1827):20160285.
Plants display extensive intraspecific variation in secondary metabolites. However, the selective forces shaping this diversity remain often unknown, especially below ground. Using Taraxacum officinale and its major native insect root herbivore Melolontha melolontha, we tested whether below-ground herbivores drive intraspecific variation in root secondary metabolites. We found that high M. melolontha infestation levels over recent decades are associated with high concentrations of major root latex secondary metabolites across 21 central European T. officinale field populations. By cultivating offspring of these populations, we show that both heritable variation and phenotypic plasticity contribute to the observed differences. Furthermore, we demonstrate that the production of the sesquiterpene lactone Taraxinic acid beta-D-glucopyranosyl ester (TA-G) is costly in the absence, but beneficial in the presence of M. melolontha, resulting in divergent selection of TA-G. Our results highlight the role of soil-dwelling insects for the evolution of plant defences in nature.
A Latex Metabolite Benefits Plant Fitness under Root Herbivore Attack.[Pubmed:26731567]
PLoS Biol. 2016 Jan 5;14(1):e1002332.
Plants produce large amounts of secondary metabolites in their shoots and roots and store them in specialized secretory structures. Although secondary metabolites and their secretory structures are commonly assumed to have a defensive function, evidence that they benefit plant fitness under herbivore attack is scarce, especially below ground. Here, we tested whether latex secondary metabolites produced by the common dandelion (Taraxacum officinale agg.) decrease the performance of its major native insect root herbivore, the larvae of the common cockchafer (Melolontha melolontha), and benefit plant vegetative and reproductive fitness under M. melolontha attack. Across 17 T. officinale genotypes screened by gas and liquid chromatography, latex concentrations of the sesquiterpene lactone Taraxinic acid beta-D-glucopyranosyl ester (TA-G) were negatively associated with M. melolontha larval growth. Adding purified TA-G to artificial diet at ecologically relevant concentrations reduced larval feeding. Silencing the germacrene A synthase ToGAS1, an enzyme that was identified to catalyze the first committed step of TA-G biosynthesis, resulted in a 90% reduction of TA-G levels and a pronounced increase in M. melolontha feeding. Transgenic, TA-G-deficient lines were preferred by M. melolontha and suffered three times more root biomass reduction than control lines. In a common garden experiment involving over 2,000 T. officinale individuals belonging to 17 different genotypes, high TA-G concentrations were associated with the maintenance of high vegetative and reproductive fitness under M. melolontha attack. Taken together, our study demonstrates that a latex secondary metabolite benefits plants under herbivore attack, a result that provides a mechanistic framework for root herbivore driven natural selection and evolution of plant defenses below ground.
Identification, quantification, spatiotemporal distribution and genetic variation of major latex secondary metabolites in the common dandelion (Taraxacum officinale agg.).[Pubmed:25682510]
Phytochemistry. 2015 Jul;115:89-98.
The secondary metabolites in the roots, leaves and flowers of the common dandelion (Taraxacum officinale agg.) have been studied in detail. However, little is known about the specific constituents of the plant's highly specialized laticifer cells. Using a combination of liquid and gas chromatography, mass spectrometry and nuclear magnetic resonance spectrometry, we identified and quantified the major secondary metabolites in the latex of different organs across different growth stages in three genotypes, and tested the activity of the metabolites against the generalist root herbivore Diabrotica balteata. We found that common dandelion latex is dominated by three classes of secondary metabolites: phenolic inositol esters (PIEs), triterpene acetates (TritAc) and the sesquiterpene lactone Taraxinic acid beta-D-glucopyranosyl ester (TA-G). Purification and absolute quantification revealed concentrations in the upper mgg(-1) range for all compound classes with up to 6% PIEs, 5% TritAc and 7% TA-G per gram latex fresh weight. Contrary to typical secondary metabolite patterns, concentrations of all three classes increased with plant age. The highest concentrations were measured in the main root. PIE profiles differed both quantitatively and qualitatively between plant genotypes, whereas TritAc and TA-G differed only quantitatively. Metabolite concentrations were positively correlated within and between the different compound classes, indicating tight biosynthetic co-regulation. Latex metabolite extracts strongly repelled D. balteata larvae, suggesting that the latex constituents are biologically active.
A new inositol triester from Taraxacum mongolicum.[Pubmed:24432736]
Nat Prod Res. 2014;28(7):420-3.
One new inositol triester, 4,5,6-tri-O-p-hydroxyphenylacetyl-chiro-inositol (1), was isolated from the ethanolic extract of Taraxacum mongolicum, along with two known compounds, 11beta,13-dihydroTaraxinic acid (2) and Taraxinic acid beta-d-glucopyranosyl ester (3). The isolates were tested for their anti-hepatitis B virus (HBV) activities; 11beta,13-dihydroTaraxinic acid (2) exhibited an IC50 value of 0.91 mM inhibiting the secretion of the HBV surface antigen and an IC50 value of 0.34 mM inhibiting the secretion of the HBV e antigen using HBV transfected Hep G2.2.15 cell line.
Sesquiterpenoids and phenolics from roots of Taraxacum udum.[Pubmed:20006977]
Fitoterapia. 2010 Jul;81(5):434-6.
From roots of Taraxacum udum, two new and four known sesquiterpene lactones were isolated, together with five known phenolic compounds. The new compounds were characterized as 11beta, 13-dihydroTaraxinic acid and Taraxinic acid 6-O-acetyl-beta-glucopyranosyl ester by spectroscopic methods, especially 1D and 2D NMR, and by comparison with structurally related compounds. The plant material was shown to be a good source of Taraxinic acid derivatives.
Sesquiterpene lactones from Taraxacum obovatum.[Pubmed:12624831]
Planta Med. 2003 Feb;69(2):181-3.
Two new guaianolide glucosides, deacetylmatricarin 8-O-beta-glucopyranoside and 11beta-hydroxyleukodin 11-O-beta-glucopyranoside, were isolated from roots of Taraxacum obovatum, along with four known sesquiterpene lactones, deacetylmatricarin, sonchuside A, Taraxinic acid beta-glucopyranosyl ester and its 11beta,13-dihydro derivative. Their structures were established by spectral methods.
[Anti-gastric ulcer sesquiterpene lactone glycosides from Crepis napifera].[Pubmed:12579896]
Yao Xue Xue Bao. 2002 Jan;37(1):33-6.
AIM: The anti-gastric ulcer constituents from the roots of Crepis napifera (Franch) Babc (Compositae) were studied. METHODS: Solvent partition, Si gel and Rp-18 column chromatography, crystallization and spectral methods were used to extract, isolate and identify two compounds. The activity of compound 1 was tested on the rat stomach by determining the effect on aspirin-induced gastric lesions and on histamine-stimulated gastric acid secretion. RESULTS: Two sesquiterpene lactone glycosides, Taraxinic acid-1'-O-beta-D-glucopyranoside (1) and 11,13-dihydro-Taraxinic acid-1'-O-beta-D-glucopyranoside (2) were obtained. Compound 1 at the dose of 80 mg.kg-1 p.o. inhibited significantly the development of aspirin-induced gastric lesions in the rat and at an i.v. dose of 70 mg.kg-1 did not affect histamine-stimulated gastric acid secretion in the lumen-perfused rat stomach. CONCLUSION: Compound 1 is the active component of the plant which protects gastric mucosa and exhibits anti-gastric ulcer action.
Taraxinic acid, a hydrolysate of sesquiterpene lactone glycoside from the Taraxacum coreanum NAKAI, induces the differentiation of human acute promyelocytic leukemia HL-60 cells.[Pubmed:12419957]
Biol Pharm Bull. 2002 Nov;25(11):1446-50.
The present work was performed to elucidate the active moiety of a sesquiterpene lactone, Taraxinic acid-1'-O-beta-D-glucopyranoside (1). from Taraxacum coreanum NAKAI on the cytotoxicity of various cancer cells. Based on enzymatic hydrolysis and MTT assay, the active moiety should be attributed to the aglycone Taraxinic acid (1a). rather than the glycoside (1). Taraxinic acid exhibited potent antiproliferative activity against human leukemia-derived HL-60. In addition, this compound was found to be a potent inducer of HL-60 cell differentiation as assessed by a nitroblue tetrazolium reduction test, esterase activity assay, phagocytic activity assay, morphology change, and expression of CD 14 and CD 66 b surface antigens. These results suggest that Taraxinic acid induces the differentiation of human leukemia cells to monocyte/macrophage lineage. Moreover, the expression level of c-myc was down-regulated during Taraxinic acid-dependent HL-60 cell differentiation, whereas p21(CIP1) and p27(KIP1) were up-regulated. Taken together, our results suggest that Taraxinic acid may have potential as a therapeutic agent in human leukemia.
Sesquiterpene glucosides from anti-leukotriene B4 release fraction of Taraxacum officinale.[Pubmed:11491394]
J Asian Nat Prod Res. 2001;3(3):191-7.
Chemical examination of the MeOH extract of the root of Taraxacum officinale, which exhibited inhibitory activity on the formation of leukotriene B4 from activated human neutrophils, has resulted in the isolation of 14-O-beta-D-glucosyl-11,13-dihydro-Taraxinic acid (1) and 14-O-beta-D-glucosyl-Taraxinic acid (2). The absolute stereostructure of 1 has been established by X-ray chrystallographic examination.


