TalcCAS# 14807-96-6 |
- Sitagliptin phosphate monohydrate
Catalog No.:BCC2111
CAS No.:654671-77-9
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14807-96-6 | SDF | Download SDF |
| PubChem ID | 16211421 | Appearance | Powder |
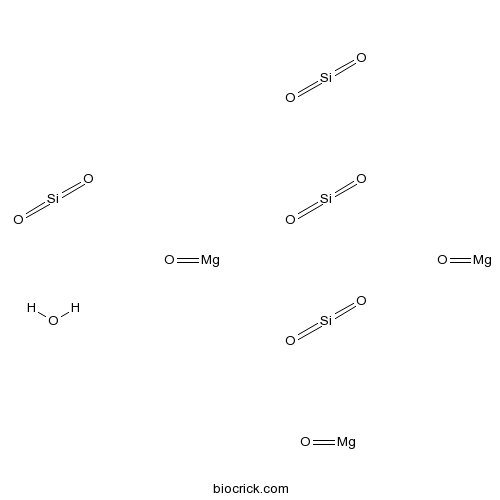
| Formula | Mg3(OH)2Si4O10 | M.Wt | 379.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | <3.8mg/mL in DMSO with gentle warming | ||
| Chemical Name | dioxosilane;oxomagnesium;hydrate | ||
| SMILES | O.O=[Mg].O=[Mg].O=[Mg].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O | ||
| Standard InChIKey | FPAFDBFIGPHWGO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/3Mg.4O2Si.H2O.3O/c;;;4*1-3-2;;;;/h;;;;;;;1H2;;; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Talc Dilution Calculator

Talc Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6366 mL | 13.1832 mL | 26.3664 mL | 52.7329 mL | 65.9161 mL |
| 5 mM | 0.5273 mL | 2.6366 mL | 5.2733 mL | 10.5466 mL | 13.1832 mL |
| 10 mM | 0.2637 mL | 1.3183 mL | 2.6366 mL | 5.2733 mL | 6.5916 mL |
| 50 mM | 0.0527 mL | 0.2637 mL | 0.5273 mL | 1.0547 mL | 1.3183 mL |
| 100 mM | 0.0264 mL | 0.1318 mL | 0.2637 mL | 0.5273 mL | 0.6592 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Talc
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- 25-Hydroxycycloart-23-en-3-one
Catalog No.:BCN1657
CAS No.:148044-47-7
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- CA-074 Me
Catalog No.:BCC3649
CAS No.:147859-80-1
- Filic-3-en-25-al
Catalog No.:BCN6445
CAS No.:147850-78-0
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
- Ac-Lys(Fmoc)-OH
Catalog No.:BCC2679
CAS No.:148101-51-3
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- (±)-Epibatidine
Catalog No.:BCC6750
CAS No.:148152-66-3
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
- Secoisolariciresinol Diglucoside
Catalog No.:BCN1212
CAS No.:148244-82-0
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- (+)-Matairesinol
Catalog No.:BCN7021
CAS No.:148409-36-3
- Prion Protein 106-126 (human)
Catalog No.:BCC6027
CAS No.:148439-49-0
- L-732,138
Catalog No.:BCC6821
CAS No.:148451-96-1
- JMV 390-1
Catalog No.:BCC5922
CAS No.:148473-36-3
Genital use of talc and risk of ovarian cancer: a meta-analysis.[Pubmed:28079603]
Eur J Cancer Prev. 2018 May;27(3):248-257.
Some epidemiological studies suggest an association between genital use of Talc powders and increased risk of ovarian cancer, but the evidence is not consistent. We performed a meta-analysis of epidemiological studies to formally evaluate this suspected association. A systematic search was conducted in Medline, Embase, and Scopus, leading to the identification of 24 case-control studies and three cohort studies. In the meta-analysis, we used a random-effect model to calculate summary estimates of the association between genital use of Talc and occurrence of ovarian cancer. We assessed potential sources of between-study heterogeneity and presence of publication bias. The summary relative risk (RR) for ever use of genital Talc and ovarian cancer was 1.22 [95% confidence interval (CI): 1.13-1.30]. The RR for case-control studies was 1.26 (95% CI: 1.17-1.35) and for cohort studies was 1.02 (95% CI: 0.85-1.20, Pheterogeneity=0.007). Serous carcinoma was the only histologic type for which an association was detected (RR: 1.24; 95% CI: 1.15-1.34). There was a weak trend in RR with duration and frequency of genital Talc use. This meta-analysis resulted in a weak but statistically significant association between genital use of Talc and ovarian cancer, which appears to be limited to serous carcinoma with suggestion of dose-response. The heterogeneity of results by study design however, detracts from a causal interpretation of this association.
Mesothelioid reaction following talc pleurodesis: a case report.[Pubmed:28271362]
Gen Thorac Cardiovasc Surg. 2017 Nov;65(11):667-669.
INTRODUCTION: Talc pleurodesis is a well-established procedure performed to obliterate the pleural space to prevent recurrent pleural effusion and/or recurrent pneumothorax. Pleurodesis is commonly accomplished by draining the pleural fluid, if present, followed by either a mechanical procedure, such as abrasion, pleurectomy, or instillation of a chemical irritant into the pleural space, which results in inflammation and fibrosis which obliterates the pleural space. The reported complications of Talc pleurodesis are hypoxemia, hypotension, tachycardia, dyspnea, chest pain, and fever. CASE PRESENTATION: Herein, we present a case of a 43-year-old female patient who developed an intense mesothelioid reaction which occurred 7 years following Talc pleurodesis. CONCLUSION: This case represents a unique mesothelioid reaction which became manifested several years after recurrent Talc pleurodesis. Such a mesothelioid reaction can clinically and radiographically mimic malignant mesothelioma. We conclude that Talc pleurodesis played a causative role in this patient developing an intense mesothelioid reaction. A mesothelioid reaction should be included in the differential diagnosis in patients with pleural thickening/pleural plaque formation who have previously been treated with Talc pleurodesis.
Lung Nodule with Increasing Fluorodeoxyglucose Uptake in a Patient with a History of Lung Carcinoma and Talc Pleurodesis Evaluated by EBUS-TBNA On-Site Assessment.[Pubmed:28110329]
Acta Cytol. 2017;61(1):84-86.
BACKGROUND: Fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET) scan is an indicator of potential malignancy or infection. Patients with a history of Talc pleurodesis can develop pleural or lung parenchymal nodules/Talcomas. In these patients, Talc-associated (non-malignancy-related) FDG uptake may occur over years. CASE REPORT: A 66-year-old female presented with a past medical history significant for resected non-small-cell lung cancer and was treated with chemotherapy/radiation. The referring physician indicated that she subsequently developed benign pleural effusions and had Talc pleurodesis to limit recurrence. The patient was referred to our institution for endobronchial ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA) due to a new left upper lobe nodule with increasing FDG uptake on follow-up interval PET performed at the referring institution. On-site cytologic evaluation showed no evidence of malignancy, but found refractile foreign material, consistent with the presence of Talc particles. CONCLUSION: This case presents the importance of cytologic recognition of Talc particles during on-site evaluation and discusses the phenomenon of increasing PET-FDG uptake associated with Talc pleurodesis.


