Taiwanin ECAS# 22743-05-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

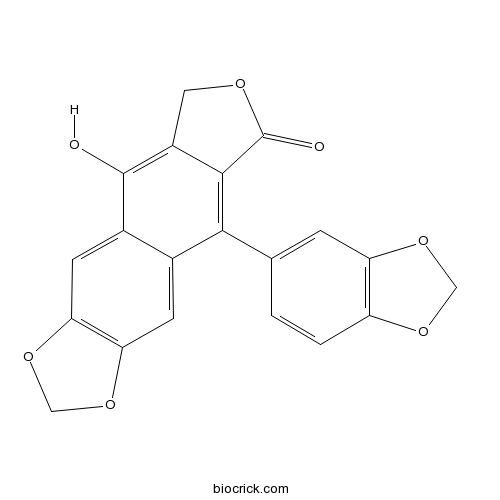
| Cas No. | 22743-05-1 | SDF | Download SDF |
| PubChem ID | 493164 | Appearance | Powder |
| Formula | C20H12O7 | M.Wt | 364.3 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 9-(1,3-benzodioxol-5-yl)-5-hydroxy-6H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one | ||
| SMILES | C1C2=C(C3=CC4=C(C=C3C(=C2C(=O)O1)C5=CC6=C(C=C5)OCO6)OCO4)O | ||
| Standard InChIKey | YYFMUDJSHVYJGD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H12O7/c21-19-11-5-16-15(26-8-27-16)4-10(11)17(18-12(19)6-23-20(18)22)9-1-2-13-14(3-9)25-7-24-13/h1-5,21H,6-8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Taiwanin E Dilution Calculator

Taiwanin E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.745 mL | 13.725 mL | 27.4499 mL | 54.8998 mL | 68.6248 mL |
| 5 mM | 0.549 mL | 2.745 mL | 5.49 mL | 10.98 mL | 13.725 mL |
| 10 mM | 0.2745 mL | 1.3725 mL | 2.745 mL | 5.49 mL | 6.8625 mL |
| 50 mM | 0.0549 mL | 0.2745 mL | 0.549 mL | 1.098 mL | 1.3725 mL |
| 100 mM | 0.0274 mL | 0.1372 mL | 0.2745 mL | 0.549 mL | 0.6862 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5-Methoxyjusticidin A
Catalog No.:BCN9611
CAS No.:205505-62-0
- 3-O-(p-Hydroxybenzoyl)serratriol
Catalog No.:BCN9610
CAS No.:1448534-93-7
- Karavilagenin F
Catalog No.:BCN9609
CAS No.:1639024-15-9
- Apigenin 7-O-(2'',6''-di-O-E-p-coumaroyl)glucoside
Catalog No.:BCN9608
CAS No.:1448779-19-8
- Beiwutine
Catalog No.:BCN9607
CAS No.:76918-93-9
- Taiwanin C
Catalog No.:BCN9606
CAS No.:14944-34-4
- Stepharine
Catalog No.:BCN9605
CAS No.:2810-21-1
- 7,7'-Dihydrotaiwanin C
Catalog No.:BCN9604
CAS No.:216955-79-2
- Menisdaurilide
Catalog No.:BCN9603
CAS No.:67765-59-7
- Aquilegiolide
Catalog No.:BCN9602
CAS No.:94481-79-5
- 16-Oxoserratenediol
Catalog No.:BCN9601
CAS No.:24513-52-8
- Norushinsunine
Catalog No.:BCN9600
CAS No.:3175-84-6
- 6,7-Di-O-acetylsinococuline
Catalog No.:BCN9613
CAS No.:1054312-81-0
- Illiciumlignan D
Catalog No.:BCN9614
CAS No.:2237239-36-8
- Tuberculatin
Catalog No.:BCN9615
CAS No.:90706-10-8
- Greveichromenol
Catalog No.:BCN9616
CAS No.:35930-29-1
- Cernuine
Catalog No.:BCN9617
CAS No.:6880-84-8
- Karavilagenin B
Catalog No.:BCN9618
CAS No.:912329-02-3
- Heteropeucenin 7-methyl ether
Catalog No.:BCN9619
CAS No.:26213-95-6
- Alloptaeroxylin
Catalog No.:BCN9620
CAS No.:4670-29-5
- 7α-O-Ethylmorroniside
Catalog No.:BCN9621
CAS No.:1116650-29-3
- O-Methylalloptaeroxylin
Catalog No.:BCN9622
CAS No.:35930-31-5
- 16-Oxo-21-episerratenediol
Catalog No.:BCN9623
CAS No.:1194739-51-9
- Umtatin
Catalog No.:BCN9624
CAS No.:17398-06-0
Taiwanin E Induces Cell Cycle Arrest and Apoptosis in Arecoline/4-NQO-Induced Oral Cancer Cells Through Modulation of the ERK Signaling Pathway.[Pubmed:31921618]
Front Oncol. 2019 Dec 17;9:1309.
Taiwanin E is a bioactive compound extracted from Taiwania cryptomerioides Hayata. In this research endeavor, we studied the anti-cancer effect of Taiwanin E against arecoline and 4-nitroquinoline-1-oxide-induced oral squamous cancer cells (OSCC), and elucidated the underlying intricacies. OSCC were treated with Taiwanin E and analyzed through MTT assay, Flow cytometry, TUNEL assay, and Western blotting for their efficacy against OSCC. Interestingly, it was found that Taiwanin E significantly attenuated the cell viability of oral cancer cells (T28); however, no significant cytotoxic effects were found for normal oral cells (N28). Further, Flow cytometry analysis showed that Taiwanin E induced G1cell cycle arrest in T28 oral cancer cells and Western blot analysis suggested that Taiwanin E considerably downregulated cell cycle regulatory proteins and activated p53, p21, and p27 proteins. Further, TUNEL and Western blot studies instigated that it induced cellular apoptosis and attenuated the p-PI3K/p-Akt survival mechanism in T28 oral cancer cells seemingly through modulation of the ERK signaling cascade. Collectively, the present study highlights the prospective therapeutic efficacy of Taiwanin E against arecoline and 4-nitroquinoline-1-oxide-induced oral cancer.
Taiwanin E inhibits cell migration in human LoVo colon cancer cells by suppressing MMP-2/9 expression via p38 MAPK pathway.[Pubmed:27807932]
Environ Toxicol. 2017 Aug;32(8):2021-2031.
Taiwanin E is a natural compound which is structurally analogous to estrogen II and is abundantly found in Taiwania cryptomerioides. It has been previously reported for its anticancer effects; however, the pharmaceutical effect of Taiwanin E on Human LoVo colon cancer cells is not clear. In this study, we investigated the effects of Taiwanin E on metastasis and the associated mechanism of action on Human LoVo colon cancer cells with respect to the modulations in their cell migration and signaling pathways associated with migration. The results showed that Taiwanin E inhibited cell migration ability correlated with reduced expression and activity of MMP-2 and MMP-9. In addition, Taiwanin E induced activation of p38 through phosphorylation. Inhibition of p38alpha/beta significantly abolished the effect of Taiwanin E on cell migration and MMP-2/-9 activity. Our results conclude that Taiwanin E inhibited cell migration chiefly via p38alpha MAPK pathway and in a lesser extend via p38beta MAPK. The results elucidate the potential of the phytoestrogen natural compound Taiwanin E as a cancer therapeutic agent in inhibiting the cell migration. (c) 2016 Wiley Periodicals, Inc. Environ Toxicol 32: 2021-2031, 2017.
Silver(I)-Catalyzed Regioselective Construction of Highly Substituted alpha-Naphthols and Its Application toward Expeditious Synthesis of Lignan Natural Products.[Pubmed:26158760]
Org Lett. 2015 Jul 17;17(14):3446-9.
A novel route has been developed for regioselective synthesis of highly substituted alpha-naphthols, binaphthols, and anthracenol through silver(I) catalyzed C(sp(3))-H/C(sp)-H, C(sp(2))-H/C(sp)-H functionalization of beta-ketoesters and alkynes, respectively, in a single step using water as a solvent. This protocol exhibited broad substrate scope and paved the way for synthesis of anticancer arylnaphthalene lignan natural products such as diphyllin, Taiwanin E, and justicidin A with excellent selectivity.
Anti-proliferation effect on human breast cancer cells via inhibition of pRb phosphorylation by taiwanin E isolated from Eleutherococcus trifoliatus.[Pubmed:25918798]
Nat Prod Commun. 2014 Sep;9(9):1303-6.
Eleutherococcus trifoliatus has been used as a folk medicine since ancient times, especially as refreshing qi medicines. In our current study, Taiwanin E, which possesses strong cytotoxicity, was isolated from the branches of E. trifoliatus by using a bioactivity guided fractionation procedure. Taiwanin E presented a potent anti-proliferation activity on the growth of a human breast adenocarcinoma cell line (MCF-7), with an IC50 value for cytotoxicity of 1.47 muM. Cell cycle analysis revealed that the proportion of cells in the G0/G1 phase increased in a dose-dependent manner (from 79.4% to 90.2%) after 48 h exposure to Taiwanin E at a dosage range from 0.5 to 4muM. After treatment with Taiwanin E, phosphorylation of retinoblastoma protein (pRb) in MCF-7 cells was inhibited, accompanied by a decrease in the levels of cyclin D1, cyclin D3 and cyclin-dependent kinase 4 (cdk4) and cdk6; in addition, there was an increase in the expression of cyclin-dependent kinase inhibitors p21(WAF-1/Cip) and p27(Kip1). The results suggest that Taiwanin E inhibits cell cycle progression of MCF-7 at the G0/G1 transition.
Evaluation and structure-activity relationship analysis of a new series of arylnaphthalene lignans as potential anti-tumor agents.[Pubmed:24675875]
PLoS One. 2014 Mar 27;9(3):e93516.
Arylnaphthalene lignan lactones have attracted considerable interest because of their anti-tumor and anti-hyperlipidimic activities. However, to our knowledge, few studies have explored the effects of these compounds on human leukemia cell lines. In this study, five arylnaphthalene lignans including 6'-hydroxy justicidin A (HJA), 6'-hydroxy justicidin B (HJB), justicidin B (JB), chinensinaphthol methyl ether (CME) and Taiwanin E methyl ether (TEME) were isolated from Justicia procumbens and their effects on the proliferation and apoptosis of the human leukemia K562 cell line were investigated then used to assess structure-activity relationships. To achieve these aims, cytotoxicity was assayed using the MTT assay, while intracellular SOD activity was detected using the SOD Activity Assay kit. Apoptosis was measured by both the using a cycle TEST PLUS DNA reagent kit as well as the FITC Annexin V apoptosis detection kit in combination with flow cytometry. Activation of caspase-mediated apoptosis was evaluated using a FITC active Caspase-3 apoptosis kit and flow cytometry. The results indicated that HJB, HJA and JB significantly inhibited the growth of K562 cells by decreasing both proliferation and SOD activity and inducing apoptosis. The sequence of anti-proliferative activity induced by the five tested arylnaphthalenes by decreasing strength was HJB > HJA > JB > CME > TEME. HJB, HJA and JB also decreased SOD activity and induced apoptosis in a dose-dependent manner. Activation of caspase-3 further indicated that HJB, HJA and JB induced caspase-dependent intrinsic and/or extrinsic apoptosis pathways. Together, these assays suggest that arylnaphthalene lignans derived from Justicia procumbens induce apoptosis to varying degrees, through a caspase-dependent pathway in human leukemia K562 cells. Furthermore, analysis of structure-activity relationships suggest that hydroxyl substitution at C-1 and C-6' significantly increased the antiproliferative activity of arylnaphthalene lignans while a methoxyl at C-1 significantly decreased the effect.
Quantification and pharmacokinetics of Taiwanin E methyl ether in rats by liquid chromatography-tandem mass spectrometry.[Pubmed:22706921]
Biomed Chromatogr. 2013 Feb;27(2):233-9.
This study firstly describes the development of an accurate and sensitive high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for the quantification of Taiwanin E methyl ether (TEME) in rat plasma. The assay involved a simple liquid-liquid extraction step with ethyl acetate and a gradient elution using a mobile phase consisting of water containing 0.1% formic acid and acetonitrile containing 0.1% formic acid. Chromatographic separation was successfully achieved on an Agilent Zorbax-C(18) column (2.1 x 50 mm, 3.5 microm) with a flow rate of 0.40 mL/min. The multiple reaction monitoring was based on the transitions of m/z = 379.1 --> 320.1 for TEME and 386.1 --> 122.0 for buspirone (internal standard). The assay was validated to demonstrate the specificity, linearity, recovery, accuracy, precision and stability. The lower limit of quantification was 0.50 ng/mL in 50 muL of rat plasma. The developed and validated method was successfully applied to the quantification and pharmacokinetic study of TEME in rats after intravenous and oral administration of 1.45 mg/kg TEME. The oral absolute bioavailability of TEME was estimated to be 5.85 +/- 1.41% with an elimination half-life value of 2.61 +/- 0.55 h, suggesting its poor absorption and/or strong metabolism in vivo.
Arylnaphthalene lignans from Taiwania cryptomerioides as novel blockers of voltage-gated K+ channels.[Pubmed:20684875]
Phytomedicine. 2010 Dec 15;18(1):46-51.
Lignans are natural phytochemicals which exhibit multiple pharmacological effects such as anti-inflammation, antivirus and anti-tumor activities. Whether they have effects on neural tissues and ion channels is still unknown. The effects of several arylnaphathalene lignans purified from Taiwania cryptomerioides on voltage-gated K(+) (Kv) channels in mouse neuroblastoma N2A cells were examined. These lignans included Taiwanin E, helioxanthin (HXT) and diphyllin. All lignans showed inhibitory effects on Kv channels and HXT was the most potent compound (IC(50)=1.7 muM). The mechanism of HXT block was further investigated. Its action was found to be extracellular but not intracellular. HXT accelerated current decay, caused a left-shift in steady-state inactivation curve but had no effect on voltage-dependence of activation. HXT block was unaffected by intracellular K(+) concentrations. Further, it did not affect ATP-sensitive K(+) channels. Our data therefore suggest that HXT is a potent and specific blocker of Kv channels, possibly with an inhibitory mechanism involving acceleration of slow inactivation.
Effects of Chamaecyparis formosensis Matasumura extractives on lipopolysaccharide-induced release of nitric oxide.[Pubmed:17291735]
Phytomedicine. 2007 Oct;14(10):675-80.
Chamaecyparis formaosensis, commonly known as Taiwan red cypress, is native to Taiwan and grows at elevations of 1500-2150 m in Taiwan's central mountains. Many compounds have been identified from different pasts of C. formosensis, but up until now, little research has been done on the link between the constituents of C. formosensis and its bioactivities. In this study, we found that an ethyl acetate fraction (EA) of methonal extract of C. formosecsis, strongly inhibited LPS-mediated nitric oxide (NO) production in Raw 264.7 cells. The EA was further divided into 25 subfractions (EA1-EA25) by column chromatography. EA12 possessed the strongest NO production inhibition activity (IC(50) was 4.1 microg/mL). At a dosage of 20 microg/mL, EA12 completely inhibited NO production and the mRNA expression of inducible nitric oxide synthase (iNOS) in LPS-stimulated macrophage RAW264.7 cells. Bioactivity-guided chromatographic fractionation and metabolite profiling coupled with spectroscopic analyses, including (1)H-NMR, (13)C-NMR analyses, identified six compounds: vanillin (1), 4-hydroxybenzaldehyde (2), trans-hinokiresinol (3), Taiwanin E (4), 4alpha-hydroxyeudesm- 11-en-12-al (5), savinin (6). All of these six compounds were the first identified and reported from this tree species. Compounds (1), (3) and (5) demonstrated significant NO inhibition effect through reduction of NO production in activated RAW 264.7 cells due to the suppression of iNOS gene expression: compounds that can selectively inhibit undesirable expression of iNOS are important as they may serve as potential cancer chemopreventatives. This study suggests that C. formosensis may have potential for use as a natural resource for human health care.
Cytotoxic arylnaphthalene lignans from a Vietnamese acanthaceae, Justicia patentiflora.[Pubmed:15921419]
J Nat Prod. 2005 May;68(5):734-8.
One new norlignan (1) and five new lignans (2-6) were isolated from the leaves and stems of Justicia patentiflora by a bioassay-guided purification. Five known compounds, carinatone, diphyllin, justicidin A, Taiwanin E, and tuberculatin, were also found in J. patentiflora. Most of the new compounds display significant activity in in vitro cytotoxic assays against KB, HCT116, and MCF-7 cancer cell lines and arrest the cell cycle in the G0/G1 phase.
Cytotoxicity of extractives from Taiwania cryptomerioides heartwood.[Pubmed:11142847]
Phytochemistry. 2000 Oct;55(3):227-32.
The cytotoxicity of the dominant lignans and sesquiterpenoids from Taiwania (Taiwania cryptomerioides Hayata) was investigated. Three human tumor cells including A-549 lung carcinoma. MCF-7 breast adenocarcinoma and HT-29 colon adenocarcinoma were selected to illustrate the structure-cytotoxicity relationships of Taiwania's dominant compounds. Taiwanin A, Taiwanin E and dimethylmatairesinol exhibited significant cytotoxicity against three human tumor cells. Among them, taiwanin A possesses the strongest cytotoxic activity. In addition, the morphology-based evaluation, flow cytometric analysis, and DNA fragmentation assays demonstrated that the tumor cell death induced by taiwanin A was due to apoptosis.
Antiplatelet arylnaphthalide lignans from Justicia procumbens.[Pubmed:8988600]
J Nat Prod. 1996 Dec;59(12):1149-50.
Fractionation of the EtOH extract of Justicia procumbens, guided by antiplatelet bioassay, led to the isolation of nine known arylnaphthalide lignans, neojusticin A (1), justicidin B (2), justicidin A (3), Taiwanin E methyl ether (4), neojusticin B (5), chinensinaphthol methyl ether (6), Taiwanin E (8), chinensinaphthol (9), and diphyllin (10), and a new arylnaphthalide lignan that was characterized by spectral means as 4'-demethylchinensinaphthol methyl ether (7). Compounds 1, 2, 4, and 8 significantly inhibited platelet aggregation.
Tandem Pummerer-Diels-Alder Reaction Sequence. A Novel Cascade Process for the Preparation of 1-Arylnaphthalene Lignans.[Pubmed:11667219]
J Org Chem. 1996 May 31;61(11):3706-3714.
The alpha-thiocarbocation generated from the Pummerer reaction of an o-benzoyl-substituted sulfoxide is intercepted by the adjacent keto group to produce an alpha-thio isobenzofuran as a transient intermediate which undergoes a subsequent Diels-Alder cycloaddition with added dienophiles. Acid-catalyzed ring-opening of the cycloadduct followed by aromatization gave an arylnaphthalene derivative. With acetylenic dienophiles, the tandem cyclization-cycloaddition sequence provided tetralones which result from a pinacol-type rearrangement of the primary cycloadducts. The versatility of the approach is highlighted through the synthesis of taiwanin C and E and justicidin E. The alpha-thiocarbocation generated from the Pummerer reaction of benzo[1,3]dioxol-5-yl-[6-[(ethylsulfinyl)methyl]benzo[1,3]dioxol-5-yl)methanone is intercepted by the adjacent keto group to produce an alpha-thioisobenzofuran as a transient intermediate which undergoes a subsequent Diels-Alder cycloaddition with dimethyl maleate. The initially formed Diels-Alder cycloadduct was readily converted to 5-benzo[1,3]dioxol-5-yl-8-(ethylthio)naphtho[2,3-d][1,3]dioxole-6,7-dicarboxylic acid dimethyl ester by loss of water on treatment with p-toluenesulfonic acid. Desulfurization of the thionaphthalene with Ra/Ni followed by hydrolysis of the less hindered methyl ester afforded 5-benzo[1,3]dioxol-5-ylnaphtho[2,3-d][1,3]dioxole-6,7-dicarboxylic acid 6-methyl ester which was further transformed into taiwanin C and justicidin E in good yield. Oxidation of the initial Diels-Alder cycloadduct with NaIO(4) in the presence of RuCl(3) followed by extrusion of ethyl sulfinate gave a naphthol derivative which can be converted into Taiwanin E.


