TCS 1205Subtype-selective GABAA receptor agonist CAS# 355022-97-8 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 355022-97-8 | SDF | Download SDF |
| PubChem ID | 10315001 | Appearance | Powder |
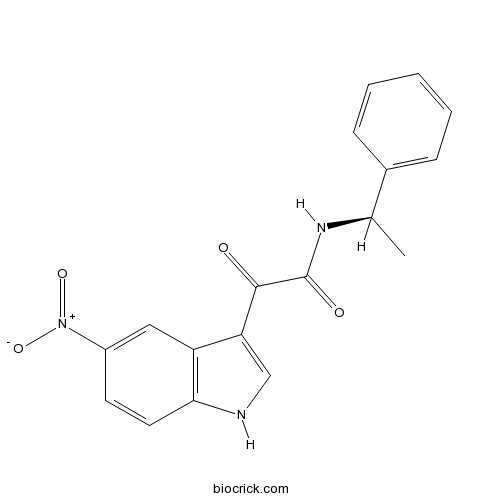
| Formula | C18H15N3O4 | M.Wt | 337.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 2-(5-nitro-1H-indol-3-yl)-2-oxo-N-[(1R)-1-phenylethyl]acetamide | ||
| SMILES | CC(C1=CC=CC=C1)NC(=O)C(=O)C2=CNC3=C2C=C(C=C3)[N+](=O)[O-] | ||
| Standard InChIKey | VCKKJKQZHHPPDR-LLVKDONJSA-N | ||
| Standard InChI | InChI=1S/C18H15N3O4/c1-11(12-5-3-2-4-6-12)20-18(23)17(22)15-10-19-16-8-7-13(21(24)25)9-14(15)16/h2-11,19H,1H3,(H,20,23)/t11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GABAA α2 agonist and GABAA α1 partial agonist in vitro (Ki values are 14 and 121 nM respectively). Exhibits non-sedative anxiolytic activity in vivo. |

TCS 1205 Dilution Calculator

TCS 1205 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9645 mL | 14.8223 mL | 29.6446 mL | 59.2891 mL | 74.1114 mL |
| 5 mM | 0.5929 mL | 2.9645 mL | 5.9289 mL | 11.8578 mL | 14.8223 mL |
| 10 mM | 0.2964 mL | 1.4822 mL | 2.9645 mL | 5.9289 mL | 7.4111 mL |
| 50 mM | 0.0593 mL | 0.2964 mL | 0.5929 mL | 1.1858 mL | 1.4822 mL |
| 100 mM | 0.0296 mL | 0.1482 mL | 0.2964 mL | 0.5929 mL | 0.7411 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-beta-O-(trans-p-Coumaroyl)maslinic acid
Catalog No.:BCN1452
CAS No.:35482-91-8
- Balicatib
Catalog No.:BCC5139
CAS No.:354813-19-7
- SC-514
Catalog No.:BCC4554
CAS No.:354812-17-2
- Corianin
Catalog No.:BCN5296
CAS No.:35481-77-7
- Hirsuteine
Catalog No.:BCN2756
CAS No.:35467-43-7
- Caraphenol A
Catalog No.:BCN5295
CAS No.:354553-35-8
- INO-1001
Catalog No.:BCC2212
CAS No.:3544-24-9
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- 5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8724
CAS No.:3543-74-6
- 1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8469
CAS No.:3543-73-5
- 1-Methyl-5-nitro-1H-benzimidazole-2-butanoic acid ethyl ester
Catalog No.:BCC8470
CAS No.:3543-72-4
- Norathyriol
Catalog No.:BCN5294
CAS No.:3542-72-1
- Ki16198
Catalog No.:BCC4560
CAS No.:355025-13-7
- Ki16425
Catalog No.:BCC1155
CAS No.:355025-24-0
- 6'-O-p-Hydroxybenzoylcatalposide
Catalog No.:BCN5297
CAS No.:355143-38-3
- YM-155 hydrochloride
Catalog No.:BCC2066
CAS No.:355406-09-6
- Buflomedil HCl
Catalog No.:BCC4760
CAS No.:35543-24-9
- N,N-dimethyl-2-Quinoxalinamine
Catalog No.:BCC9066
CAS No.:35552-76-2
- tert-Butyl rosuvastatin
Catalog No.:BCC9163
CAS No.:355806-00-7
- Betmidin
Catalog No.:BCN8253
CAS No.:35589-22-1
- Fluocinonide
Catalog No.:BCC4953
CAS No.:356-12-7
- Darapladib
Catalog No.:BCC1515
CAS No.:356057-34-6
- Toceranib
Catalog No.:BCC2005
CAS No.:356068-94-5
- N-Desethyl Sunitinib
Catalog No.:BCC1792
CAS No.:356068-97-8
Identification of anxiolytic/nonsedative agents among indol-3-ylglyoxylamides acting as functionally selective agonists at the gamma-aminobutyric acid-A (GABAA) alpha2 benzodiazepine receptor.[Pubmed:19469479]
J Med Chem. 2009 Jun 25;52(12):3723-34.
Anxioselective agents may be identified among compounds binding selectively to the alpha(2)beta(x)gamma(2) subtype of the gamma-aminobutyric acid-A (GABA(A))/central benzodiazepine receptor (BzR) complex and behaving as agonists or among compounds binding with comparable potency to various BzR subtypes but eliciting agonism only at the alpha(2)beta(x)gamma(2) receptor. Because of subtle steric differences among BzR subtypes, the latter approach has proved much more successful. A biological screening within the class of indol-3-ylglyoxylamides 1-3 allowed us to identify compounds 1c and 2b as potential anxiolytic/nonsedative agents showing alpha(2) selective efficacy in vitro and anxioselective effects in vivo. According to molecular modeling studies, and consistently with SARs accumulated in the past decade, 5-NO(2)- and 5-H-indole derivatives would preferentially bind to BzR by placing the indole ring in the L(Di) and the L(2) receptor binding sites, respectively.
Novel N-(arylalkyl)indol-3-ylglyoxylylamides targeted as ligands of the benzodiazepine receptor: synthesis, biological evaluation, and molecular modeling analysis of the structure-activity relationships.[Pubmed:11428922]
J Med Chem. 2001 Jul 5;44(14):2286-97.
A series of N-(arylalkyl)indol-3-ylglyoxylylamides (4-8) was synthesized as ligands of the benzodiazepine receptor (BzR) and tested for their ability to displace [(3)H]flumazenil from bovine brain membranes. The new compounds, bearing a branched (4) or a geometrically constrained benzyl/phenylethyl amide side chain (5-8), represent the continuation of our research on N-benzylindol-3-ylglyoxylylamides 1 (Da Settimo et al., 1996), N'-phenylindol-3-ylglyoxylohydrazides 2 (Da Settimo et al., 1998), and N-(indol-3-ylglyoxylyl)alanine derivatives 3 (Primofiore et al., 1989). A few indoles belonging to the previously investigated benzylamides 1 and phenylhydrazides 2 were synthesized and tested to enrich the SARs in these two series. The affinities and the GABA ratios of selected compounds for clonal mammalian alpha(1)beta(2)gamma(2), alpha(3)beta(2)gamma(2), and alpha(5)beta(3)gamma(2) BzR subtypes were also determined. It was hypothesized that the reduced flexibility of indoles 4-8 would both facilitate the mapping of the BzR binding cleft and increase the chances of conferring selectivity for the considered receptor subtypes. In the series of indoles 4, the introduction of a methyl group on the benzylic carbon with the R configuration improved affinity of the 5-substituted (5-Cl and 5-NO(2)) derivatives, whereas it was detrimental for their 5-unsubtituted (5-H) counterparts. All S enantiomers were less potent than the R ones. Replacement of the methyl with hydrophilic substituents on the benzylic carbon lowered affinity. The isoindolinylamide side chain was tolerated if the 5-position was unsubstituted (K(i) of 5a = 123 nM), otherwise affinity was abolished (5b, c). All the 2-indanylamides 6 and (S)-1-indanylamides 8 were devoid of any appreciable affinity. The 5-Cl and 5-NO(2) (R)-1-indanylamides 7b (K(i) 80 nM) and 7c (K(i) 28 nM) were the most potent among the indoles 5-8 geometrically constrained about the side chain. The 5-H (R)-1-indanylamide 7a displayed a lower affinity (K(i) 675 nM). The SARs developed from the new compounds, together with those collected from our previous studies, confirmed the hypothesis of different binding modes for 5-substituted and 5-unsubstituted indoles, suggesting that the shape of the lipophilic pocket L(1) (notation in accordance with Cook's BzR topological model) is asymmetric and highlighted the stereoelectronic and conformational properties of the amide side chain required for high potency. Several of the new indoles showed selectivity for the alpha(1)beta(2)gamma(2) subtype compared with the alpha(3)beta(2)gamma(2) and alpha(5)beta(3)gamma(2) subtypes (e.g.: 4t and 7c bind to these three BzR isoforms with K(i) values of 14 nM, 283 nM, 239 nM, and 9 nM, 1960 nM, 95 nM, respectively). The GABA ratios close to unity exhibited by all the tested compounds on each BzR subtype were predictive of an efficacy profile typical of antagonists.


