SulfathiazoleCAS# 72-14-0 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 72-14-0 | SDF | Download SDF |
| PubChem ID | 5340 | Appearance | Powder |
| Formula | C9H9N3O2S2 | M.Wt | 255.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
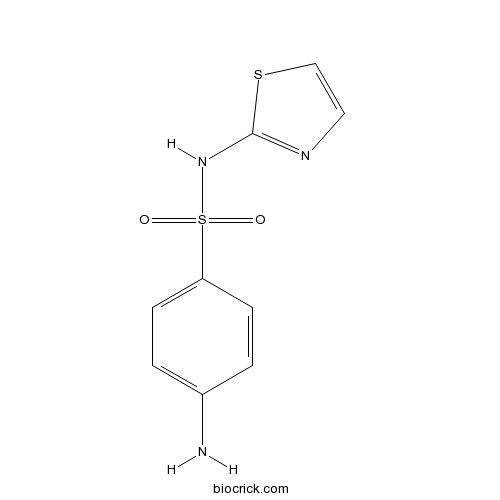
| Chemical Name | 4-amino-N-(1,3-thiazol-2-yl)benzenesulfonamide | ||
| SMILES | C1=CC(=CC=C1N)S(=O)(=O)NC2=NC=CS2 | ||
| Standard InChIKey | JNMRHUJNCSQMMB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H9N3O2S2/c10-7-1-3-8(4-2-7)16(13,14)12-9-11-5-6-15-9/h1-6H,10H2,(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sulfathiazole is an organosulfur compound that has been used as a short-acting sulfa drug.
Target: Antibacterial
Sulfathiazole (20 μg/L) starts to be degraded between day 31 and day 38 in one of the two batch reactors containing different wastewater matrices. Sulfathiazole is degraded at a substantially faster rate than sulfamethoxazole or sulfamethazine in the nitrification process (S3) [1]. Recovery from spiked manure slurry samples is 64% for Sulfathiazole at pH 9. Sulfathiazole has acidity constant of pKa of 7.1and retention times (tR) of 7.8. S/N values for Sulfathiazole are above 100 at the 1 mg/kg level [2]. Sulfathiazole sorption to inorganic sorbents exhibits pronounced pH dependence consistent with sorbate speciation and sorbent charge properties. Sulfathiazole cations are most important for sorption to clay minerals, followed by neutral species [3]. References: | |||||

Sulfathiazole Dilution Calculator

Sulfathiazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9167 mL | 19.5833 mL | 39.1665 mL | 78.3331 mL | 97.9163 mL |
| 5 mM | 0.7833 mL | 3.9167 mL | 7.8333 mL | 15.6666 mL | 19.5833 mL |
| 10 mM | 0.3917 mL | 1.9583 mL | 3.9167 mL | 7.8333 mL | 9.7916 mL |
| 50 mM | 0.0783 mL | 0.3917 mL | 0.7833 mL | 1.5667 mL | 1.9583 mL |
| 100 mM | 0.0392 mL | 0.1958 mL | 0.3917 mL | 0.7833 mL | 0.9792 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sulfathiazole is an organosulfur compound that has been used as a short-acting sulfa drug.
- H-Thr(tBu)-OMe.HCl
Catalog No.:BCC3107
CAS No.:71989-43-0
- Fmoc-Tyr(Bzl)-OH
Catalog No.:BCC3564
CAS No.:71989-40-7
- Fmoc-Tyr(tBu)-OH
Catalog No.:BCC3567
CAS No.:71989-38-3
- Fmoc-Thr(tBu)-OH
Catalog No.:BCC3552
CAS No.:71989-35-0
- Fmoc-Ser(tBu)-OH
Catalog No.:BCC3544
CAS No.:71989-33-8
- Fmoc-Pro-OH
Catalog No.:BCC3538
CAS No.:71989-31-6
- Fmoc-Met-OH
Catalog No.:BCC3528
CAS No.:71989-28-1
- Fmoc-Lys(Boc)-OH
Catalog No.:BCC3516
CAS No.:71989-26-9
- Fmoc-Ile-OH
Catalog No.:BCC3505
CAS No.:71989-23-6
- Fmoc-Gln-OH
Catalog No.:BCC3483
CAS No.:71989-20-3
- Fmoc-Glu(OtBu)-OH
Catalog No.:BCC3494
CAS No.:71989-18-9
- Fmoc-Asn-OH
Catalog No.:BCC3079
CAS No.:71989-16-7
- H-Val-OH
Catalog No.:BCC3138
CAS No.:72-18-4
- H-Thr-OH
Catalog No.:BCC3102
CAS No.:72-19-5
- Alizarin
Catalog No.:BCN3479
CAS No.:72-48-0
- 2,2-Bis(4-chlorophenyl)-1,1-dichloroethane
Catalog No.:BCC8492
CAS No.:72-54-8
- 2,2-Bis(4-chlorophenyl)-1,1-dichloroethylene
Catalog No.:BCC8493
CAS No.:72-55-9
- Metandienone
Catalog No.:BCC9025
CAS No.:72-63-9
- Chlorquinaldol
Catalog No.:BCC4648
CAS No.:72-80-0
- 2-Geranyl-4-isobutyrylphloroglucinol
Catalog No.:BCN7170
CAS No.:72008-03-8
- Henryoside
Catalog No.:BCN4276
CAS No.:72021-23-9
- NHS-LC-Biotin
Catalog No.:BCC3579
CAS No.:72040-63-2
- Lochnericine
Catalog No.:BCN4595
CAS No.:72058-36-7
- Sepinol
Catalog No.:BCN4277
CAS No.:72061-63-3
Peculiar behavior of starch 2,3-dialdehyde towards sulfanilamide and sulfathiazole.[Pubmed:27516312]
Carbohydr Polym. 2016 Nov 5;152:624-631.
In this study, starch (1) was oxidized to starch-2,3-dialdehyde (DAS; 2) using potassium periodate. In addition, two novel Schiff's bases (5 &6) were synthesized via a condensation reaction between DAS (2) and sulfa drugs (sulfanilamide; 3 & Sulfathiazole; 4). The synthesized Schiff's bases (5 &6) were characterized by FT-IR spectroscopy, X-ray diffraction and DSC analysis. DAS can easily be oxidized owing to its high aldehyde content (91.0%). However, it has low reactivity towards sulfanilamide (3) and Sulfathiazole (4). According to the diffraction functional theory, this peculiar behavior is caused by the absence of V-shape in alpha-glucan linkage in DAS molecules, making the carbonyl group least electropositive. This reduces the nucleophilic attacks of the amino group in sulfa drugs towards the carbonyl group in DAS.
Evaluation of sulfathiazole degradation by persulfate in Milli-Q water and in effluent of a sewage treatment plant.[Pubmed:27287494]
Environ Sci Pollut Res Int. 2017 Mar;24(7):6270-6277.
The presence of antibiotics and their metabolites in natural waters has raised some concern among scientists around the world because it can lead to bacterial resistance and other unknown consequences to mankind and wildlife. Persulfate (PS)-driven oxidation is a new technology that has been used successfully to remediate contaminated sites, but its use to treat wastewater, especially sewage treatment plant (STP) effluent, is still scarce. This paper describes the effect of several persulfate activation methods for degrading Sulfathiazole (STZ) in Milli-Q water and in STP effluent. Some parameters, such as pH, persulfate concentration, presence of Mn(2+), Zn(2+), Cu(2+), Fe(2+), and Fe(3+), as well as copper and iron organic complexes, were studied in STZ degradation. Raising the pH from 5 to 9, as well as the persulfate concentration, resulted in increased STZ degradation. Among the transition metals evaluated, only Fe(2+) and Cu(2+) were able to activate persulfate molecules. Copper was a better activator than iron since its effect lasts longer. Citrate was the best ligand evaluated increasing Fe(II) activation capacity at pH 7. Hydroxylamine addition to Fe(II) on persulfate system extended the Fe(II) effect. The presence of bicarbonate or humic acid did not affect PS-driven degradation of STZ. Finally, the degradation of STZ in STP effluent promoted by PS-driven oxidation (25 degrees C) was as fast as in Milli-Q water, proving to be successful.
Structural characterization of new Schiff bases of sulfamethoxazole and sulfathiazole, their antibacterial activity and docking computation with DHPS protein structure.[Pubmed:26056977]
Spectrochim Acta A Mol Biomol Spectrosc. 2015;150:268-79.
New Schiff bases (1, 2) of substituted salicylaldehydes and sulfamethoxazole (SMX)/Sulfathiazole (STZ) are synthesized and characterized by elemental analysis and spectroscopic data. Single crystal X-ray structure of one of the compounds (E)-4-((3,5-dichloro-2-hydroxybenzylidene)amino)-N-(5-methylisoxazol-3-yl)benzene sulfonamide (1c) has been determined. Antimicrobial activities of the Schiff bases and parent sulfonamides (SMX, STZ) have been examined against several Gram-positive and Gram-negative bacteria and sulfonamide resistant pathogens; the lowest MIC is observed for (E)-4-((3,5-dichloro-2-hydroxybenzylidene)amino)-N-(thiazol-2-yl)benzene sulfonamide (2c) (8.0 mug mL(-1)) and (E)-4-((3,5-dichloro-2-hydroxybenzylidene)amino)-N-(5-methylisoxazol-3-yl)benzene sulfonamide (1c) (16.0 mug mL(-1)) against sulfonamide resistant pathogens. DFT optimized structures of the Schiff bases have been used to carry out molecular docking studies with DHPS (dihydropteroate synthase) protein structure (downloaded from Protein Data Bank) using Discovery Studio 3.5 to find the most preferred binding mode of the ligand inside the protein cavity. The theoretical data have been well correlated with the experimental results. Cell viability assay and ADMET studies predict that 1c and 2c have good drug like characters.
A Drug-Drug Salt Hydrate of Norfloxacin and Sulfathiazole: Enhancement of in Vitro Biological Properties via Improved Physicochemical Properties.[Pubmed:27580175]
Mol Pharm. 2016 Oct 3;13(10):3590-3594.
A new multicomponent solid consisting of an antibacterial (norfloxacin) and an antimicrobial (Sulfathiazole) was made and characterized with single crystal X-ray diffraction, PXRD, FTIR, and DSC. The title salt shows enhanced solubility in different pH buffers and improved diffusion as well as release and inhibition of bacterial and fungal species relative to the parent drugs. The enhanced in vitro biological properties of the drug-drug salt hydrate may be attributed to the higher extent of its supersaturation with respect to the individual components, which leads to higher diffusion rates.


