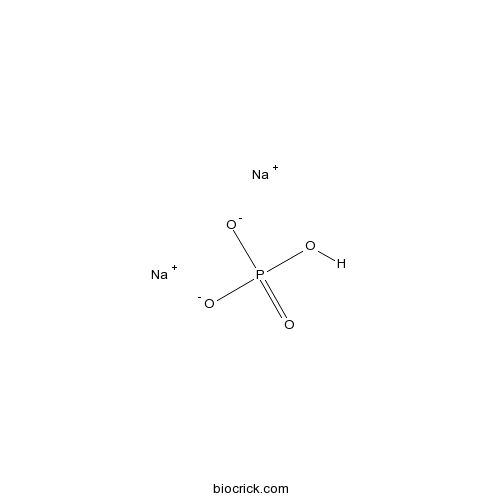
Sodium phosphate dibasicCAS# 7558-79-4 |
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
- Tasquinimod
Catalog No.:BCC1987
CAS No.:254964-60-8
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7558-79-4 | SDF | Download SDF |
| PubChem ID | 24203 | Appearance | Powder |
| Formula | Na2HPO4 | M.Wt | 141.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 300 mM in water | ||
| Chemical Name | disodium;hydrogen phosphate | ||
| SMILES | OP(=O)([O-])[O-].[Na+].[Na+] | ||
| Standard InChIKey | BNIILDVGGAEEIG-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/2Na.H3O4P/c;;1-5(2,3)4/h;;(H3,1,2,3,4)/q2*+1;/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Commonly used in biological assay buffers. |

Sodium phosphate dibasic Dilution Calculator

Sodium phosphate dibasic Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.0442 mL | 35.2212 mL | 70.4424 mL | 140.8848 mL | 176.1059 mL |
| 5 mM | 1.4088 mL | 7.0442 mL | 14.0885 mL | 28.177 mL | 35.2212 mL |
| 10 mM | 0.7044 mL | 3.5221 mL | 7.0442 mL | 14.0885 mL | 17.6106 mL |
| 50 mM | 0.1409 mL | 0.7044 mL | 1.4088 mL | 2.8177 mL | 3.5221 mL |
| 100 mM | 0.0704 mL | 0.3522 mL | 0.7044 mL | 1.4088 mL | 1.7611 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 20-Deoxyingenol 3-angelate
Catalog No.:BCN6642
CAS No.:75567-38-3
- Ingenol 3-Angelate
Catalog No.:BCN2961
CAS No.:75567-37-2
- Dencichin
Catalog No.:BCN2555
CAS No.:7554-90-7
- Moxonidine hydrochloride
Catalog No.:BCC5163
CAS No.:75536-04-8
- Nilvadipine
Catalog No.:BCC3799
CAS No.:75530-68-6
- Cedrin
Catalog No.:BCN4748
CAS No.:75513-81-4
- Flupirtine maleate
Catalog No.:BCC4456
CAS No.:75507-68-5
- BI6727 (Volasertib)
Catalog No.:BCC3886
CAS No.:755038-65-4
- BI 2536
Catalog No.:BCC2081
CAS No.:755038-02-9
- Regorafenib
Catalog No.:BCC3646
CAS No.:755037-03-7
- N-Methylnuciferine
Catalog No.:BCN3971
CAS No.:754919-24-9
- CGP 37157
Catalog No.:BCC6943
CAS No.:75450-34-9
- Sodium phosphate monobasic
Catalog No.:BCC8033
CAS No.:7558-80-7
- alpha-Tocopherolquinone
Catalog No.:BCN4305
CAS No.:7559-04-8
- Kaerophyllin
Catalog No.:BCN4304
CAS No.:75590-33-9
- Methyl 3,4,5-trimethoxycinnamate
Catalog No.:BCN4589
CAS No.:7560-49-8
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- (S)-(+)-α-Methylhistamine dihydrobromide
Catalog No.:BCC6700
CAS No.:75614-93-6
- (-)-Usnic acid
Catalog No.:BCN4306
CAS No.:7562-61-0
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
- Moracenin B
Catalog No.:BCC8341
CAS No.:75629-19-5
- Gomisin K1
Catalog No.:BCN7030
CAS No.:75629-20-8
- Oncrasin 1
Catalog No.:BCC2390
CAS No.:75629-57-1
- Knightolamine
Catalog No.:BCN1912
CAS No.:75638-70-9
Response of rainbow trout (Oncorhynchus mykiss) growing from 50 to 200 g to supplements of dibasic sodium phosphate in a semipurified diet.[Pubmed:8558318]
J Nutr. 1996 Jan;126(1):324-31.
Effects of increasing dietary concentrations of phosphorus on growth, feed intake, feed conversion, composition of gain and concentration of inorganic phosphate in plasma were studied in rainbow trout. Twelve groups of 20 trout initially weighing 53 +/- 0.6 g/fish were fed semipurified diets containing 19.6 MJ digestible energy per kilogram of dry matter. Twelve levels of phosphorus ranging from 1.03 to 10.96 g/kg dry matter were achieved by replacing inorganic binder with dibasic sodium phosphate in 11 graded levels. Nonlinear responses of trout to increasing dietary phosphorus concentration determined over 53 d were described using exponential functions. Feed intake, growth rate and feed conversion ratio as well as plasma inorganic phosphate concentration increased with increasing dietary phosphorus concentration. The concentrations of phosphorus, calcium, magnesium and potassium in weight gain increased, whereas concentrations of lipids and energy in weight gain decreased with increasing dietary phosphorus concentration. Concentrations of protein and sodium in weight gain were unaffected. Different concentrations of dietary phosphorus were required for achieving 95% of the plateau value determined for desired traits. In growth rate and phosphorus deposition, the required phosphorus concentrations were 3.7 and 5.6 g/kg dry matter, respectively. However, dietary phosphorus was utilized most efficiently (88%) at a dietary concentration of 2.5 g/kg dry matter. At the dietary phosphorus concentration that resulted in maximum phosphorus deposition (5.6 g/kg dry matter), phosphorus utilization was about 60%. Supplemental phosphorus from dibasic sodium phosphate was completely available to trout which must be considered in formulating recommendations. Based on this work, 0.25 g available phosphorus/MJ digestible energy is recommended for trout diets.
Nephrotic syndrome induced by dibasic sodium phosphate injections for twenty-eight days in rats.[Pubmed:19244217]
Toxicol Pathol. 2009 Apr;37(3):270-9.
Sprague-Dawley rats received once daily tail-vein injections of 360 mM dibasic sodium phosphate solution at 8 mL/kg for fourteen or twenty-eight days. Clinical examination revealed persistent proteinuria from three days after the first dosing and thereafter severe proteinuria from eight days or later in the phosphate-treated groups. Proteinuria developed without remission even after fourteen-day withdrawal in the fourteen-day dosed group. Phosphate-treated animals developed lipemia, hypercholesterolemia, anemia, higher serum fibrinogen levels, and lower serum albumin/globulin ratios on day 29. Renal weight increased significantly compared with control animals, and the kidneys appeared pale and enlarged with a rough surface. Histopathologically, glomerular changes consisted of mineralization in whole glomeruli, glomerular capillary dilatation, partial adhesion of glomerular tufts to Bowman's capsule, and mesangiolysis. Ultrastructural lesions such as an increased number of microvilli, effacement of foot processes, and thickening of the glomerular basement membrane, and immunocytochemical changes in podocytes, mainly decreased podoplanin-positive cells and increased desmin expression, were also conspicuous in the phosphate-treated rats for twenty-eight days. Marked tubulointerstitial lesions were tubular regeneration and dilatation, protein casts, mineralization in the basement membrane, focal interstitial inflammation, and fibrosis in the cortex. These clinical and morphological changes were similar to features of human nephrotic syndrome.
Preparation and evaluation of sodium diclofenac controlled-release tablets. II. Dibasic calcium phosphate as a retardant in mixtures for direct compression.[Pubmed:7795557]
Pharm World Sci. 1995 Mar 24;17(2):42-7.
The dissolution behaviour of a direct compression compact prepared with sodium diclofenac and dibasic calcium phosphate (DCP) in different weight ratios with or without Biosoluble polymer (acrylic-based resin) was investigated in distilled water and in a medium with changing pH. The results indicate that the amount of sodium diclofenac released from the compact was dependent on the amount of drug and DCP used in the compact, and was also controlled by the amount of Biosoluble polymer added. A chemical reaction forming diclofenac acid might occur on the surface of the sodium diclofenac compact during exposure to the acidic medium, which was confirmed by diffuse-reflectance spectroscopy. The tablet with a 1:2 weight ratio of sodium diclofenac to DCP exhibited a sustained-release behaviour, similar to commercial sustained-release products (Voltaren SR-100 and Grofenac Retard), but a lower release rate was found as compared to the commercial products. The dissolution behaviour of the study tablet and the commercial products was found to be dependent on the dissolution medium and the rotating speeds. Glass beads were added to the dissolution assembly to simulate the influence of food, and the enhanced friction between tablet and glass beads might result in a higher dissolution rate of the tablet and the commercial products.
Glomerular calcification induced by bolus injection with dibasic sodium phosphate solution in Sprague-Dawley rats.[Pubmed:15204963]
Toxicol Pathol. 2004 Jul-Aug;32(4):408-12.
To elucidate the nephrotoxicity of phosphate, dibasic sodium phosphate solution was given to Sprague-Dawley rats by daily bolus intravenous administration at concentrations of 0, 1, 25, 250, or 360 mM (0, 1, 28, 284, or 408 mg/kg Na2HPO4) for 14 days, and the kidneys were pathologically examined. There were no remarkable changes in blood chemistry values; however, urinalysis revealed mild to moderate proteinuria in the 250 and 360 mM groups. The kidneys from the 360 mM group were macroscopically pale. Histopathology revealed panglomerular deposition of basophilic dense granules, which were positive for von Kossa's staining, accompanied by dose-dependent degeneration of the glomerular epithelium and parietal epithelium in the 250 and 360 mM groups. Electron microscopic examination showed fusion of podocytes and increased microvilli, with large amounts of debris in the Bowman's space. Low-density lamellar structures were present not only in the glomerular epithelium, basement membrane, mesangial matrix and parietal epithelium but also within the Bowman's space depending on the severity of the glomerular lesion. Phosphorus and calcium were detected by X-ray microanalysis as fine particles admixed with lamellar structures. These results suggest that high-dose phosphate used in this study transiently overloads the glomerular epithelium during filtration through glomerular capillaries and produces insoluble calcium salt and glomerular lesions, resulting in proteinuria.


