Sideroxylonal ACAS# 145382-68-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 145382-68-9 | SDF | Download SDF |
| PubChem ID | 10391273 | Appearance | Powder |
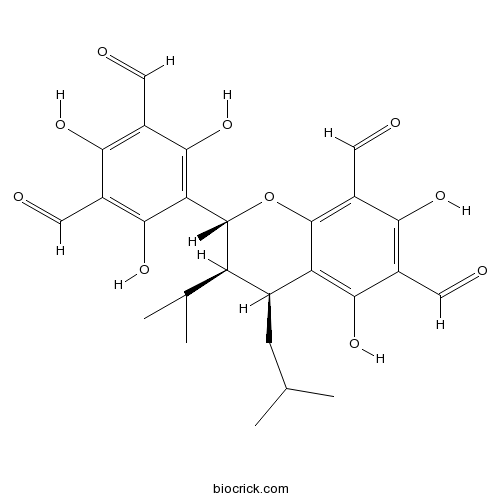
| Formula | C26H28O10 | M.Wt | 500.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,4S)-2-(3,5-diformyl-2,4,6-trihydroxyphenyl)-5,7-dihydroxy-4-(2-methylpropyl)-3-propan-2-yl-3,4-dihydro-2H-chromene-6,8-dicarbaldehyde | ||
| SMILES | CC(C)CC1C(C(OC2=C(C(=C(C(=C12)O)C=O)O)C=O)C3=C(C(=C(C(=C3O)C=O)O)C=O)O)C(C)C | ||
| Standard InChIKey | PHQDMQGEKNBIPF-FLFOAQQMSA-N | ||
| Standard InChI | InChI=1S/C26H28O10/c1-10(2)5-12-17(11(3)4)26(19-23(34)13(6-27)20(31)14(7-28)24(19)35)36-25-16(9-30)21(32)15(8-29)22(33)18(12)25/h6-12,17,26,31-35H,5H2,1-4H3/t12-,17+,26+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Sideroxylonal A, isolated from the benzene extract of the leaves of Eucalyptus grandis, is a highly potent repellent. |

Sideroxylonal A Dilution Calculator

Sideroxylonal A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.998 mL | 9.99 mL | 19.98 mL | 39.96 mL | 49.95 mL |
| 5 mM | 0.3996 mL | 1.998 mL | 3.996 mL | 7.992 mL | 9.99 mL |
| 10 mM | 0.1998 mL | 0.999 mL | 1.998 mL | 3.996 mL | 4.995 mL |
| 50 mM | 0.04 mL | 0.1998 mL | 0.3996 mL | 0.7992 mL | 0.999 mL |
| 100 mM | 0.02 mL | 0.0999 mL | 0.1998 mL | 0.3996 mL | 0.4995 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isochlorogenic acid B
Catalog No.:BCN5909
CAS No.:14534-61-3
- Dihydropinosylvin
Catalog No.:BCN6258
CAS No.:14531-52-3
- Bergenin pentaacetate
Catalog No.:BCN6257
CAS No.:14531-47-6
- TC-E 5002
Catalog No.:BCC5608
CAS No.:1453071-47-0
- A 77636 hydrochloride
Catalog No.:BCC7159
CAS No.:145307-34-2
- 20(R)-Protopanaxatriol
Catalog No.:BCN1079
CAS No.:1453-93-6
- Diarylcomosol III
Catalog No.:BCN7201
CAS No.:1452487-93-2
- Sahandol
Catalog No.:BCN6996
CAS No.:1452398-07-0
- Delgrandine
Catalog No.:BCN8122
CAS No.:145237-05-4
- Clobenpropit dihydrobromide
Catalog No.:BCC6781
CAS No.:145231-35-2
- CALP1
Catalog No.:BCC5873
CAS No.:145224-99-3
- Punctanecine
Catalog No.:BCN2018
CAS No.:145204-91-7
- GDC-0994
Catalog No.:BCC6371
CAS No.:1453848-26-4
- 1,4,7-Eudesmanetriol
Catalog No.:BCN1646
CAS No.:145400-02-8
- Homalomenol A
Catalog No.:BCN1647
CAS No.:145400-03-9
- L-692,585
Catalog No.:BCC7305
CAS No.:145455-35-2
- Heterophyllin B
Catalog No.:BCN2768
CAS No.:145459-19-4
- Isolintetralin
Catalog No.:BCN3052
CAS No.:145459-30-9
- PU-WS13
Catalog No.:BCC6425
CAS No.:1454619-14-7
- Furano(2'',3'',7,6)-4'-hydroxyflavanone
Catalog No.:BCN6405
CAS No.:1454619-70-5
- LY3009120
Catalog No.:BCC3985
CAS No.:1454682-72-4
- 4-Benzoylpyridine
Catalog No.:BCC8697
CAS No.:14548-46-0
- PF-06463922
Catalog No.:BCC5568
CAS No.:1454846-35-5
- MRS 2179 tetrasodium salt
Catalog No.:BCC5685
CAS No.:1454889-37-2
Sideroxylonal C, a new inhibitor of human plasminogen activator inhibitor type-1, from the flowers of Eucalyptus albens.[Pubmed:10075775]
J Nat Prod. 1999 Feb;62(2):324-6.
Sideroxylonal C (3), a new phloroglucinol dimer, was isolated from the flowers of Eucalyptus albens through bioassay-guided fractionation. The structure elucidation was based on 1D and 2D NMR experiments, MS analysis, and comparison with sideroxylonals A (1) and B (2). Sideroxylonal C inhibited human plasminogen activator inhibitor type-1 at 4.7 microM without any significant effect on human tissue plasminogen activator.
Chemical variation in a dominant tree species: population divergence, selection and genetic stability across environments.[Pubmed:23526981]
PLoS One. 2013;8(3):e58416.
Understanding among and within population genetic variation of ecologically important plant traits provides insight into the potential evolutionary processes affecting those traits. The strength and consistency of selection driving variability in traits would be affected by plasticity in differences among genotypes across environments (GxE). We investigated population divergence, selection and environmental plasticity of foliar plant secondary metabolites (PSMs) in a dominant tree species, Eucalyptus globulus. Using two common garden trials we examined variation in PSMs at multiple genetic scales; among 12 populations covering the full geographic range of the species and among up to 60 families within populations. Significant genetic variation in the expression of many PSMs resides both among and within populations of E. globulus with moderate (e.g., Sideroxylonal A h(2)op = 0.24) to high (e.g., macrocarpal G h(2)op = 0.48) narrow sense heritabilities and high coefficients of additive genetic variation estimated for some compounds. A comparison of Qst and Fst estimates suggest that variability in some of these traits may be due to selection. Importantly, there was no genetic by environment interaction in the expression of any of the quantitative chemical traits despite often significant site effects. These results provide evidence that natural selection has contributed to population divergence in PSMs in E. globulus, and identifies the formylated phloroglucinol compounds (particularly sideroxylonal) and a dominant oil, 1,8-cineole, as candidates for traits whose genetic architecture has been shaped by divergent selection. Additionally, as the genetic differences in these PSMs that influence community phenotypes is stable across environments, the role of plant genotype in structuring communities is strengthened and these genotypic differences may be relatively stable under global environmental changes.


