Salvianolic acid FCAS# 158732-59-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 158732-59-3 | SDF | Download SDF |
| PubChem ID | 10903113 | Appearance | Powder |
| Formula | C17H14O6 | M.Wt | 314.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
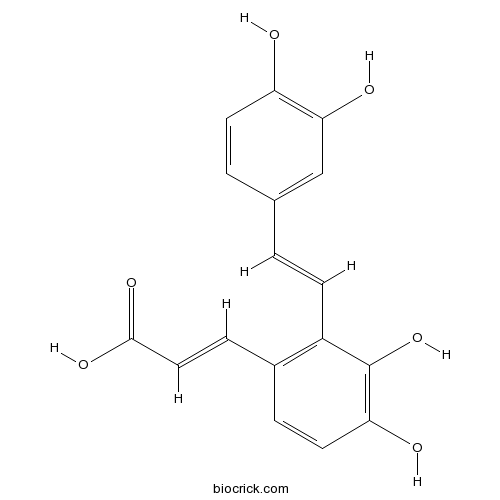
| Chemical Name | (E)-3-[2-[(E)-2-(3,4-dihydroxyphenyl)ethenyl]-3,4-dihydroxyphenyl]prop-2-enoic acid | ||
| SMILES | C1=CC(=C(C=C1C=CC2=C(C=CC(=C2O)O)C=CC(=O)O)O)O | ||
| Standard InChIKey | PULWRMOKQNWQBD-LZSLGQGWSA-N | ||
| Standard InChI | InChI=1S/C17H14O6/c18-13-6-2-10(9-15(13)20)1-5-12-11(4-8-16(21)22)3-7-14(19)17(12)23/h1-9,18-20,23H,(H,21,22)/b5-1+,8-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | Caspase |

Salvianolic acid F Dilution Calculator

Salvianolic acid F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1817 mL | 15.9084 mL | 31.8167 mL | 63.6335 mL | 79.5418 mL |
| 5 mM | 0.6363 mL | 3.1817 mL | 6.3633 mL | 12.7267 mL | 15.9084 mL |
| 10 mM | 0.3182 mL | 1.5908 mL | 3.1817 mL | 6.3633 mL | 7.9542 mL |
| 50 mM | 0.0636 mL | 0.3182 mL | 0.6363 mL | 1.2727 mL | 1.5908 mL |
| 100 mM | 0.0318 mL | 0.1591 mL | 0.3182 mL | 0.6363 mL | 0.7954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aescin IIA
Catalog No.:BCN6551
CAS No.:158732-55-9
- 2-Naphthyl N-benzoylphenylalaninate
Catalog No.:BCC8583
CAS No.:15873-25-3
- Dihydrexidine hydrochloride
Catalog No.:BCC5681
CAS No.:158704-02-0
- Rimonabant hydrochloride
Catalog No.:BCC1898
CAS No.:158681-13-1
- Ombuin 3-glucoside
Catalog No.:BCN4055
CAS No.:158642-42-3
- Eclalbasaponin I
Catalog No.:BCN8244
CAS No.:158511-59-2
- Cnidioside B methyl ester
Catalog No.:BCN1707
CAS No.:158500-59-5
- Catalpalactone
Catalog No.:BCN1708
CAS No.:1585-68-8
- ω-Agatoxin TK
Catalog No.:BCC7489
CAS No.:158484-42-5
- BMS-983970
Catalog No.:BCC5509
CAS No.:1584713-87-0
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Demethyl calyciphylline A
Catalog No.:BCN7040
CAS No.:1584236-34-9
- Escin IIB
Catalog No.:BCN8127
CAS No.:158800-83-0
- GR 159897
Catalog No.:BCC7001
CAS No.:158848-32-9
- 3-O-(2'E ,4'Z-decadienoyl)-20-O-acetylingenol
Catalog No.:BCN1550
CAS No.:158850-76-1
- Boc-ß-HoAla-OH
Catalog No.:BCC3224
CAS No.:158851-30-0
- 3Alaph-Tigloyloxypterokaurene L3
Catalog No.:BCN6787
CAS No.:1588516-87-3
- ent-17-Hydroxykaura-9(11),15-dien-19-oic acid
Catalog No.:BCN6788
CAS No.:1588516-88-4
- BET-BAY 002
Catalog No.:BCC5510
CAS No.:1588521-78-1
- GR 231118
Catalog No.:BCC7085
CAS No.:158859-98-4
- APC 366
Catalog No.:BCC7392
CAS No.:158921-85-8
- Boc-D-Tryptophanol
Catalog No.:BCC2698
CAS No.:158932-00-4
- Secoisolarisiresinol Diglucoside
Catalog No.:BCC9140
CAS No.:158932-33-3
- Wedelobatin A
Catalog No.:BCN6731
CAS No.:1589488-34-5
Styryl-cinnamate hybrid inhibits glioma by alleviating translation, bioenergetics and other key cellular responses leading to apoptosis.[Pubmed:30513337]
Exp Cell Res. 2019 Feb 1;375(1):11-21.
Gliomas are lethal and aggressive form of brain tumors with resistance to conventional radiation and cytotoxic chemotherapies; inviting continuous efforts for drug discovery and drug delivery. Interestingly, small molecule hybrids are one such pharmacophore that continues to capture interest owing to their pluripotent medicinal effects. Accordingly, we earlier reported synthesis of potent Styryl-cinnamate hybrids (analogues of Salvianolic acid F) along with its plausible mode of action (MOA). We explored iTRAQ-LC/MS-MS technique to deduce differentially expressed landscape of native & phospho-proteins in treated glioma cells. Based on this, Protein-Protein Interactome (PPI) was looked into by employing computational tools and further validated in vitro. We hereby report that the Styryl-cinnamate hybrid, an analogue of natural Salvianolic acid F, alters key regulatory proteins involved in translation, cytoskeleton development, bioenergetics, DNA repair, angiogenesis and ubiquitination. Cell cycle analysis dictates arrest at G0/G1 stage along with reduced levels of cyclin D; involved in G1 progression. We discovered that Styryl-cinnamate hybrid targets glioma by intrinsically triggering metabolite-mediated stress. Various oncological circuits alleviated by the potential drug candidate strongly supports the role of such pharmacophores as anticancer drugs. Although, further analysis of SC hybrid in treating xenografts or solid tumors is yet to be explored but their candidature has gained huge impetus through this study. This study equips us better in understanding the shift in proteomic landscape after treating glioma cells with SC hybrid. It also allows us to elicit molecular targets of this potential drug before progressing to preclinical studies.
Multiple on-line screening and identification methods for hydroxyl radical scavengers in Yudanshen.[Pubmed:29730337]
J Pharm Biomed Anal. 2018 Jul 15;156:278-283.
Yudanshen, the genuine medicinal materials of Danshen (Salvia miltiorrhiza), is a well-known traditional Chinese medicine (TCM) used to treat cardiovascular and cerebrovascular diseases. Although its pharmacological and antioxidative activities have been well-documented, there is little research on the hydroxyl radical (OH) scavenging capacity of Yudanshen. In this study, we established multiple on-line high-performance liquid chromatography- chemiluminescence detector-diode-quadrupole-time of flight mass spectrometry (HPLC-CL-DAD-Q-TOF/MS) methods to rapidly screen and identify the OH scavengers in Yudanshen simultaneously. The chromatographic and potency fingerprints revealed seventeen peaks that showed the inhibition of OH. Fourteen of them were identified as danshensu, protocatechuic aldehyde, caffeic acid, ferulic acid, Salvianolic acid F, salvianolic acid H/L, salvianolic acid G, salvianolic acid D, salvianolic acid E, rosmarinic acid, salvianolic acid B, isosalvianolic acid B, salvianolic acid A, and salvianolic acid C. This study explores the OH scavenging activities of Yudanshen, and provides novel and powerful multiple on-line methods in the field of TCM for rapid screening and identification of OH scavengers.


