SMIFH2FH2 domain inhibitor; prevents actin nucleation CAS# 340316-62-3 |
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- LKB1 (AAK1 dual inhibitor)
Catalog No.:BCC1705
CAS No.:1093222-27-5
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- CX-6258 hydrochloride hydrate
Catalog No.:BCC1505
CAS No.:1353858-99-7
- PIM-1 Inhibitor 2
Catalog No.:BCC2446
CAS No.:477845-12-8
- TCS PIM-1 1
Catalog No.:BCC2447
CAS No.:491871-58-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 340316-62-3 | SDF | Download SDF |
| PubChem ID | 2258538 | Appearance | Powder |
| Formula | C15H9BrN2O3S | M.Wt | 377.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
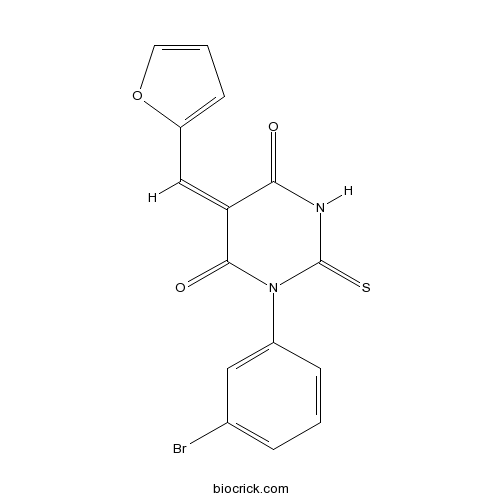
| Chemical Name | (5E)-1-(3-bromophenyl)-5-(furan-2-ylmethylidene)-2-sulfanylidene-1,3-diazinane-4,6-dione | ||
| SMILES | C1=CC(=CC(=C1)Br)N2C(=O)C(=CC3=CC=CO3)C(=O)NC2=S | ||
| Standard InChIKey | MVFJHEQDISFYIS-XYOKQWHBSA-N | ||
| Standard InChI | InChI=1S/C15H9BrN2O3S/c16-9-3-1-4-10(7-9)18-14(20)12(13(19)17-15(18)22)8-11-5-2-6-21-11/h1-8H,(H,17,19,22)/b12-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of formin homology 2 (FH2) domains. Prevents formin-mediated actin nucleation and barbed end elongation. Disrupts formin-dependent actin cytoskeletal structures in fission yeast and mammalian NIH 3T3 fibroblasts. |

SMIFH2 Dilution Calculator

SMIFH2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.651 mL | 13.2552 mL | 26.5104 mL | 53.0209 mL | 66.2761 mL |
| 5 mM | 0.5302 mL | 2.651 mL | 5.3021 mL | 10.6042 mL | 13.2552 mL |
| 10 mM | 0.2651 mL | 1.3255 mL | 2.651 mL | 5.3021 mL | 6.6276 mL |
| 50 mM | 0.053 mL | 0.2651 mL | 0.5302 mL | 1.0604 mL | 1.3255 mL |
| 100 mM | 0.0265 mL | 0.1326 mL | 0.2651 mL | 0.5302 mL | 0.6628 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Auranofin
Catalog No.:BCC7952
CAS No.:34031-32-8
- H-D-Phe-OtBu.HCl
Catalog No.:BCC3014
CAS No.:3403-25-6
- 1-BCP
Catalog No.:BCC6909
CAS No.:34023-62-6
- Vinorine
Catalog No.:BCN4649
CAS No.:34020-07-0
- Z-Ala-OSu
Catalog No.:BCC3057
CAS No.:3401-36-3
- 7-Oxodehydroabietinol
Catalog No.:BCN5265
CAS No.:33980-71-1
- Clivorine
Catalog No.:BCN2067
CAS No.:33979-15-6
- Pelargonidin-3-O-rutinosde chloride
Catalog No.:BCN3112
CAS No.:33978-17-5
- Triacetonamine hydrochloride
Catalog No.:BCN5264
CAS No.:33973-59-0
- Z-Leu-Osu
Catalog No.:BCC2592
CAS No.:3397-35-1
- H-D-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3174
CAS No.:33965-47-8
- Thalrugosidine
Catalog No.:BCN7785
CAS No.:33954-34-6
- Diclofensine hydrochloride
Catalog No.:BCC5541
CAS No.:34041-84-4
- 1,3-Bis(4,5-dihydro-2-oxazolyl)benzene
Catalog No.:BCC8417
CAS No.:34052-90-9
- 2-2'-(Hydroxytetracosanoylamino)-octadecane-1,3,4-triol tetraacetate
Catalog No.:BCN1454
CAS No.:340702-68-3
- Z-Pro-NH2
Catalog No.:BCC2753
CAS No.:34079-31-7
- (20S)-Protopanaxatriol
Catalog No.:BCN2705
CAS No.:34080-08-5
- Alpinumisoflavone
Catalog No.:BCN5266
CAS No.:34086-50-5
- 7-O-Methylbiochanin A
Catalog No.:BCN8212
CAS No.:34086-51-6
- Glycyrrhetic acid 3-O-mono-beta-D-glucuronide
Catalog No.:BCN1453
CAS No.:34096-83-8
- Araloside V
Catalog No.:BCN2466
CAS No.:340963-86-2
- Fmoc-D-Asp-OtBu
Catalog No.:BCC3470
CAS No.:34098-70-7
- Araloside VII
Catalog No.:BCN8129
CAS No.:340982-22-1
- Nucleozin
Catalog No.:BCC1811
CAS No.:341001-38-5
SMIFH2-mediated mDia formin functional inhibition potentiates chemotherapeutic targeting of human ovarian cancer spheroids.[Pubmed:26898799]
Biochem Biophys Res Commun. 2016 Mar 25;472(1):33-9.
Due to a lack of effective screening or prevention protocol for epithelial ovarian cancer (EOC), there is a critical unmet need to develop therapeutic interventions for EOC treatment. EOC metastasis is unique. Initial dissemination is not primarily hematogenous, yet is facilitated through shedding of primary tumor cells into the peritoneal fluid and accumulating ascites. Increasingly, isolated patient spheroids point to a clinical role for spheroids in EOC metastasis. EOC spheroids are highly invasive structures that disseminate upon peritoneal mesothelium, and visceral tissues including liver and omentum. Selection for this subset of chemoresistant EOC cells could influence disease progression and/or recurrence. Thus, targeting spheroid integrity/structure may improve the chemotherapeutic responsiveness of EOC. We discovered a critical role for mammalian Diaphanous (mDia)-related formin-2 in maintaining EOC spheroid structure. Both mDia2 and the related mDia1 regulate F-actin networks critical to maintain cell-cell contacts and the integrity of multi-cellular epithelial sheets. We investigated if mDia2 functional inhibition via a small molecule inhibitor SMIFH2 combined with chemotherapeutics, such as taxol and cisplatin, inhibits the viability of EOC monolayers and clinically relevant spheroids. SMIFH2-mediated mDia formin inhibition significantly reduced both ES2 and Skov3 EOC monolayer viability while spheroid viability was minimally impacted only at the highest concentrations. Combining either cisplatin or taxol with SMIFH2 did not significantly enhance the effects of either drug alone in ES2 monolayers, while Skov3 monolayers treated with taxol or cisplatin and SMIFH2 showed significant additive inhibition of viability. ES2 spheroids were highly responsive with clear additive anti-viability effects with dual taxol or cisplatin when combined with SMIFH2 treatments. While combined taxol with SMIFH2 in spheroids showed an additive effect relative to single treatments, Skov3 spheroids showed no additive effects from combined cisplatin and SMIFH2 treatments. Our data indicate that mDia formin inhibition combined with taxol to drive enhanced and/or additive anti-viability effects targeting 3D EOC structures, including ES2 and Skov3 spheroids. Combined mDia formin inhibition with cisplatin may be most effective in EOC spheroids where cisplatin sensitivity is retained at moderate levels, such as ES2 cells.
Small molecule inhibitor of formin homology 2 domains (SMIFH2) reveals the roles of the formin family of proteins in spindle assembly and asymmetric division in mouse oocytes.[Pubmed:25837661]
PLoS One. 2015 Apr 2;10(4):e0123438.
Dynamic actin reorganization is the main driving force for spindle migration and asymmetric cell division in mammalian oocytes. It has been reported that various actin nucleators including Formin-2 are involved in the polarization of the spindle and in asymmetric cell division. In mammals, the formin family is comprised of 15 proteins. However, their individual roles in spindle migration and/or asymmetric division have not been elucidated yet. In this study, we employed a newly developed inhibitor for formin family proteins, small molecule inhibitor of formin homology 2 domains (SMIFH2), to assess the functions of the formin family in mouse oocyte maturation. Treatment with SMIFH2 during in vitro maturation of mouse oocytes inhibited maturation by decreasing cytoplasmic and cortical actin levels. In addition, treatment with SMIFH2, especially at higher concentrations (500 muM), impaired the proper formation of meiotic spindles, indicating that formins play a role in meiotic spindle formation. Knockdown of the mDia2 formins caused a similar decrease in oocyte maturation and abnormal spindle morphology, mimicking the phenotype of SMIFH2-treated cells. Collectively, these results suggested that besides Formin-2, the other proteins of the formin, including mDia family play a role in asymmetric division and meiotic spindle formation in mammalian oocytes.
SMIFH2 has effects on Formins and p53 that perturb the cell cytoskeleton.[Pubmed:25925024]
Sci Rep. 2015 Apr 30;5:9802.
Formin proteins are key regulators of the cytoskeleton involved in developmental and homeostatic programs, and human disease. For these reasons, small molecules interfering with Formins' activity have gained increasing attention. Among them, small molecule inhibitor of Formin Homology 2 domains (SMIFH2) is often used as a pharmacological Formin blocker. Although SMIFH2 inhibits actin polymerization by Formins and affects the actin cytoskeleton, its cellular mechanism of action and target specificity remain unclear. Here we show that SMIFH2 induces remodelling of actin filaments, microtubules and the Golgi complex as a result of its effects on Formins and p53. We found that SMIFH2 triggers alternated depolymerization-repolymerization cycles of actin and tubulin, increases cell migration, causes scattering of the Golgi complex, and also cytotoxicity at high dose. Moreover, SMIFH2 reduces expression and activity of p53 through a post-transcriptional, proteasome-independent mechanism that influences remodelling of the cytoskeleton. As the action of SMIFH2 may go beyond Formin inhibition, only short-term and low-dose SMIFH2 treatments minimize confounding effects induced by loss of p53 and cytotoxicity.
Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel.[Pubmed:21245302]
Proc Natl Acad Sci U S A. 2011 Feb 1;108(5):1943-8.
Cancer cells use different modes of migration, including integrin-dependent mesenchymal migration of elongated cells along elements of the 3D matrix as opposed to low-adhesion-, contraction-based amoeboid motility of rounded cells. We report that MDA-MB-231 human breast adenocarcinoma cells invade 3D Matrigel with a characteristic rounded morphology and with F-actin and myosin-IIa accumulating at the cell rear in a uropod-like structure. MDA-MB-231 cells display neither lamellipodia nor bleb extensions at the leading edge and do not require Arp2/3 complex activity for 3D invasion in Matrigel. Accumulation of phospho-MLC and blebbing activity were restricted to the uropod as reporters of actomyosin contractility, and velocimetric analysis of fluorescent beads embedded within the 3D matrix showed that pulling forces exerted to the matrix are restricted to the side and rear of cells. Inhibition of actomyosin contractility or beta1 integrin function interferes with uropod formation, matrix deformation, and invasion through Matrigel. These findings support a model whereby actomyosin-based uropod contractility generates traction forces on the beta1 integrin adhesion system to drive cell propulsion within the 3D matrix, with no contribution of lamellipodia extension or blebbing to movement.
Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly.[Pubmed:19942139]
Chem Biol. 2009 Nov 25;16(11):1158-68.
Formins stimulate actin filament assembly for fundamental cellular processes including division, adhesion, establishing polarity, and motility. A formin inhibitor would be useful because most cells express multiple formins whose functions are not known and because metastatic tumor formation depends on the deregulation of formin-dependent processes. We identified a general small molecule inhibitor of formin homology 2 domains (SMIFH2) by screening compounds for the ability to prevent formin-mediated actin assembly in vitro. SMIFH2 targets formins from evolutionarily diverse organisms including yeast, nematode worm, and mice, with a half-maximal inhibitor concentration of approximately 5 to 15 microM. SMIFH2 prevents both formin nucleation and processive barbed end elongation and decreases formin's affinity for the barbed end. Furthermore, low micromolar concentrations of SMIFH2 disrupt formin-dependent, but not Arp2/3 complex-dependent, actin cytoskeletal structures in fission yeast and mammalian NIH 3T3 fibroblasts.


