SDZ 21009CAS# 39731-05-0 |
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
- A 83-01
Catalog No.:BCC1319
CAS No.:909910-43-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 39731-05-0 | SDF | Download SDF |
| PubChem ID | 193949 | Appearance | Powder |
| Formula | C19H28N2O4 | M.Wt | 348.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
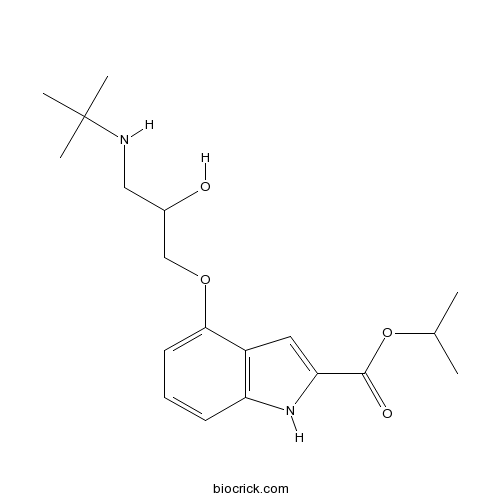
| Chemical Name | propan-2-yl 4-[3-(tert-butylamino)-2-hydroxypropoxy]-1H-indole-2-carboxylate | ||
| SMILES | CC(C)OC(=O)C1=CC2=C(N1)C=CC=C2OCC(CNC(C)(C)C)O | ||
| Standard InChIKey | SJYFDORQYYEJLB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H28N2O4/c1-12(2)25-18(23)16-9-14-15(21-16)7-6-8-17(14)24-11-13(22)10-20-19(3,4)5/h6-9,12-13,20-22H,10-11H2,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | β-adrenoceptor and 5-HT1A/1B receptor antagonist. pKB/pA2 values are 8.3 and 8.0 for 5-HT1A and 5-HT1B receptors respectively. Centrally active following systemic administration in vivo. |

SDZ 21009 Dilution Calculator

SDZ 21009 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8699 mL | 14.3497 mL | 28.6993 mL | 57.3987 mL | 71.7484 mL |
| 5 mM | 0.574 mL | 2.8699 mL | 5.7399 mL | 11.4797 mL | 14.3497 mL |
| 10 mM | 0.287 mL | 1.435 mL | 2.8699 mL | 5.7399 mL | 7.1748 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.574 mL | 1.148 mL | 1.435 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.574 mL | 0.7175 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gue 1654
Catalog No.:BCC6274
CAS No.:397290-30-1
- Daphmacropodine
Catalog No.:BCN5450
CAS No.:39729-21-0
- Catharticin
Catalog No.:BCN6850
CAS No.:39723-40-5
- 2-Benzylsuccinic acid
Catalog No.:BCC8566
CAS No.:3972-36-9
- 1,5-Diphenylpentan-1-one
Catalog No.:BCN7169
CAS No.:39686-51-6
- (R)-Reticuline
Catalog No.:BCN6795
CAS No.:3968-19-2
- BVT 948
Catalog No.:BCC2467
CAS No.:39674-97-0
- Azatadine
Catalog No.:BCC4133
CAS No.:3964-81-6
- Isoshinanolone
Catalog No.:BCN7986
CAS No.:39626-91-0
- LY364947
Catalog No.:BCC5085
CAS No.:396129-53-6
- Pasireotide
Catalog No.:BCC5300
CAS No.:396091-73-9
- Z-Sar-OH
Catalog No.:BCC3339
CAS No.:39608-31-6
- H-Gln-OtBu.HCl
Catalog No.:BCC2918
CAS No.:39741-62-3
- 16,16-Dimethyl Prostaglandin E2
Catalog No.:BCC7843
CAS No.:39746-25-3
- Dehydrovomifoliol
Catalog No.:BCN7562
CAS No.:39763-33-2
- Bryonolol
Catalog No.:BCN2703
CAS No.:39765-50-9
- 2-Amino-4-hydroxy-6-methylpyrimidine
Catalog No.:BCC8531
CAS No.:3977-29-5
- Boc-Tyr-OH
Catalog No.:BCC3458
CAS No.:3978-80-1
- Azatadine dimaleate
Catalog No.:BCC4536
CAS No.:3978-86-7
- Penciclovir
Catalog No.:BCC4695
CAS No.:39809-25-1
- Taibaihenryiins A
Catalog No.:BCN3281
CAS No.:398129-59-4
- Epitulipinolide diepoxide
Catalog No.:BCN5451
CAS No.:39815-40-2
- H-Ala-OiPr.HCl
Catalog No.:BCC3193
CAS No.:39825-33-7
- Amikacin disulfate
Catalog No.:BCC4622
CAS No.:39831-55-5
5-HT(1B) but not 5-HT(6) or 5-HT(7) receptors mediate depression of spinal nociceptive reflexes in vitro.[Pubmed:11861321]
Br J Pharmacol. 2002 Feb;135(4):935-42.
1. The identity of the serotonin (5-HT) receptors modulating the transmission of segmental C-fibre mediated signals was studied using an in vitro preparation of the hemisected spinal cord from rat pups. 2. Responses to trains of stimuli delivered to a lumbar dorsal root were recorded from the corresponding ventral root. The resulting cumulative depolarization (CD) mediated by unmyelinated fibres was quantified in terms of integrated area. The amplitude of the mono-synaptic reflex was also measured. Serotonergic agents were superfused at known concentrations and their effects on the reflexes evaluated. 3. 5-HT had depressant effects on the CD (EC(50) 34 microM). The rank order of potency of agonists for the depression of the CD was 5-carboxamidotryptamine (5-CT)>alpha-methylserotonin (alpha-met-5-HT) approximately 5-HT>42-methylserotonin (2-met-5-HT)approximately 8-OH-DPAT. 4. All the agonists including 2-met-5-HT and 8-OH-DPAT had strong depressant effects on the mono-synaptic reflex with the following order of potency: 5-CT>48-OH-DPAT>4alpha-met-5-HT approximate5-HTapproximate2-met-5-HT. 5. The inhibitory effects of 5-HT, alpha-met-5-HT and 5-CT were attenuated by the non-specific 5-HT antagonist methiothepin (1 microM) and by the 5-HT(1A/1B) antagonist SDZ 21009 (100 nM) but not by the selective 5-HT(1A) antagonist WAY 100135 (1 microM). 6. Other antagonists known to block 5-HT(2), 5-HT(6) and/or 5-HT(7) receptors (ketanserin, RO 04-6790, ritanserin and clozapine) did not change the effect of the agonists. 7. The data suggest an important contribution of 5-HT(1B) receptors to the inhibition of spinal C-fibre mediated nociceptive reflexes but no experimental support was found for the intervention of 5-HT(2), 5-HT(6) or 5-HT(7) receptors in this in vitro model.
Behavioral effect of beta-blocking drugs resulting from the stimulation or the blockade of serotonergic 5-HT1B receptors.[Pubmed:7972302]
Pharmacol Biochem Behav. 1994 Aug;48(4):965-9.
The present study was aimed at determining the relative potency of various beta-blocking drugs as agonists or antagonists at 5-HT1B receptors. The behavioral model used (increase in escape attempts of isolated mice) has been previously shown to be exclusively responsive to 5-HT1B agonists such as 1-3-(trifluoromethyl) phenylpiperazine (TFMPP). Beta-blocking drugs acted in three different ways: they were either inactive, or acted as agonists or as antagonists at 5-HT1B receptors. The specific beta-blocking drugs: atenolol and betaxolol (beta-1) and ICI 118,551 (beta-2) were inactive by themselves and in interaction with TFMPP. The mixed beta-1 beta-2 blocking drug 1-penbutolol, (but not d-penbutolol), inactive alone, behaved as an antagonist: it impaired in a dose-dependent way the effect of TFMPP. (+/-)Pindolol and (-)pindolol was inactive. None of the (-), (+), or (+/-)pindolol was able to impair TFMPP effect. The increase in escape attempts induced by (+/-)pindolol was antagonized with 1-penbutolol or after a specific desensitization. Cyanopindolol and S-tertatolol (but not R-tertatolol) acted as agonists. SDZ 21009 was inactive as agonist or antagonist. It may be concluded that all beta-blocking drugs are not equivalent regarding their effect at 5-HT1B receptors. L-penbutolol was the only drug acting as an antagonist.
Non 5-HT1A/5-HT1C [3H]5-HT binding sites in the hamster, opossum, and rabbit brain show similar regional distribution but different sensitivity to beta-adrenoceptor antagonists.[Pubmed:1361246]
Synapse. 1992 Dec;12(4):261-70.
We have used receptor autoradiography to investigate the distribution and pharmacological profile of non 5-HT1A/5-HT1C[3H]5-hydroxytryptamine binding sites in the brain of rabbits, hamsters and opossums. These data were compared to those found under similar conditions in the brain of rats and guinea pigs, species which are known to possess 5-HT1B and 5-HT1D receptors, respectively. In the presence of 100 nM 8-OH-DPAT and mesulergine, the regional distribution of [3H]5-hydroxytryptamine binding sites was very similar in the brain of all species investigated; densest labelling was observed in the globus pallidus, substantia nigra and superior colliculus. In all species, 5-carboxamidotryptamine competed for the labelled sites in a biphasic manner and metergoline displayed a subnanomolar affinity. In contrast, the beta-adrenoceptor blocking agents (-)propranolol, (-)pindolol, and (+/-)SDZ 21009 were potent displacers only in the rat, hamster and opossum brains. These data indicate that non 5-HT1A/5-HT1C[3H]5-HT binding sites display a high affinity for these agents in a particular rodent suborder as well as in opossum, a phylogenetically unrelated species.
Involvement of dopamine autoreceptors in the hypoactivity induced by 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in mice.[Pubmed:1357637]
Pol J Pharmacol Pharm. 1992 Mar-Apr;44(2):135-46.
The effect of 8-OH-DPAT, a 5-HT1A receptor agonist, on the locomotor activity was analyzed in Albino Swiss mice. The studied drug (0.5-5 mg/kg) inhibited the spontaneous locomotor activity in mice. The hypoactivity induced by 8-OH-DPAT (1.5 mg/kg) was abolished by the dopamine (D1 and D2) receptor antagonist-haloperidol (0.00125 and 0.0025 mg/kg, but not in higher doses) and by the D2 antagonist with affinity for 5-HT1A and 5-HT2 receptors-spiperone (0.0025 and 0.005 mg/kg, but not in higher doses). The effect of 8-OH-DPAT was slightly reduced by the alpha 2-adrenoceptor antagonists: idazoxan (4 mg/kg), yohimbine (2 and 4 mg/kg) and rauwolscine (4 mg/kg). On the other hand, the non-selective 5-HT antagonist metergoline (0.5-4 mg/kg), the 5-HT1A antagonist NAN-190 (0.5-2 mg/kg), the beta-adrenoceptor blockers with high affinity for 5-HT1A and 5-HT1B receptors: pindolol and SDZ 21009 (2-8 mg/kg) and the agonist/antagonist of 5-HT1A receptors ipsapirone (2.5 and 5 mg/kg) did not affect the 8-OH-DPAT-induced hypoactivity. The obtained results suggest that the reduction of the spontaneous locomotor activity induced by 8-OH-DPAT results from a stimulation of dopamine autoreceptors, but not 5-HT receptors. Involvement of an alpha 2-adrenergic mechanism cannot be excluded.
Involvement of 5-HT1B receptors in the anticonflict effect of m-CPP in rats.[Pubmed:1348421]
J Neural Transm Gen Sect. 1992;87(2):87-96.
The anticonflict activity of m-CPP, a non-selective agonist of 5-HT receptors, was studied in the drinking conflict test in rats. m-CPP administered in doses of 0.125-0.5 mg/kg increased the number of punished licks, the maximum effect having been observed after a dose of 0.25 mg/kg. The anticonflict effect of m-CPP (0.25 mg/kg) was antagonized by the non-selective 5-HT antagonist metergoline (1-4 mg/kg) and by the beta-adrenoceptor blocker SDZ 21009 (2 and 4 mg/kg) with affinity for 5-HT1A and 5-HT1B receptors. On the other hand, the 5-HT1A receptor antagonist NAN-190 (0.5 and 1 mg/kg), the 5-HT2 receptor antagonist ritanserin (0.25 and 0.5 mg/kg), and the beta-blockers betaxolol (8 mg/kg) and ICI 118,551 (8 mg/kg) with no affinity for 5-HT receptors did not affect the effect of m-CPP. The effect of m-CPP was not modified, either, in animals with the 5-HT lesion produced by p-chloroamphetamine. These results suggest that the anticonflict effect of m-CPP described above results from stimulation of 5-HT1B receptors--most probably these which are located postsynaptically.
Evidence for the involvement of 5-HT1A receptors in the anticonflict effect of ipsapirone in rats.[Pubmed:1681448]
Neuropharmacology. 1991 Jul;30(7):703-9.
The anticonflict activity of ipsapirone, a non-benzodiazepine anxiolytic drug, with high affinity for 5-hydroxytryptamine1A (5-HT1A) receptors, was studied in the drinking conflict test in the rat. The drug, administered in doses 1.25-20 mg/kg increased the number of punished licks, with the maximum effect observed after doses of 5-20 mg/kg of ipsapirone. The anticonflict effect of ipsapirone (5 mg/kg) was dose-dependently antagonized by the 5-HT1A receptor, alpha 1-adrenoceptor and dopamine receptor antagonist, NAN-190 (0.25-1 mg/kg) and by the beta-adrenoceptor blocker, SDZ 21009, which also has a high affinity for 5-HT1A and 5-HT1B receptors (2-8 mg/kg). On the other hand, the non-selective 5-HT receptor antagonist, metergoline (2 and 4 mg/kg), the 5-HT2/5-HT1C receptor antagonist, ritanserin (0.25 and 0.5 mg/kg), the selective alpha 1-adrenoceptor blocker, prazosin (0.25-0.5 mg/kg) and the beta-blockers, betaxolol (8 mg/kg) and ICI 118 551 (8 mg/kg), which have no affinity for 5-HT receptors, did not affect the anticonflict action of ipsapirone. The effect of ipsapirone was also not modified in animals with lesions of 5-HT neurones, produced by p-chloroamphetamine (PCA--2 x 10 mg/kg). These results suggest that the anticonflict effect of ipsapirone in the Vogel test, results from its interaction with 5-HT1A receptors, which are probably located postsynaptically.
Inhibition of noradrenaline release from the sympathetic nerves of the human saphenous vein via presynaptic 5-HT receptors similar to the 5-HT 1D subtype.[Pubmed:2255330]
Naunyn Schmiedebergs Arch Pharmacol. 1990 Oct;342(4):371-7.
The human saphenous vein preincubated with [3H]noradrenaline was used to determine the pharmacological properties of the release-inhibiting presynaptic serotonin (5-HT) receptor on the sympathetic nerves. The overflow of tritium evoked by transmural electrical stimulation (2 Hz) was concentration-dependently inhibited by drugs known to stimulate 5-HT receptors in the following rank order: oxymetazoline greater than or equal to 5-HT greater than 5-carboxamidotryptamine = 5-methoxytryptamine = sumatriptan greater than tryptamine greater than N,N(CH3)2-5-HT = yohimbine = 8-hydroxy-2-(di-n-propylamino)-tetraline. The potencies of these agonists in inhibiting overflow were significantly correlated with their affinities for 5-HT1B and 5-HT1D binding sites, but not with those for 5-HT1A or 5-HT1C binding sites. 5-Aminotryptamine, methysergide, ipsapirone, cyanopindolol, SDZ 21009 and metergoline dit not produce a significant inhibition. Metitepine and methysergide antagonized the inhibitory effect of 5-HT, whereas spiroxatrine, propranolol, ketanserin and ICS 205-930 did not. These data exclude the idea that the inhibitory presynaptic 5-HT receptor on the sympathetic nerves belongs to the 5-HT2 and 5-HT3 receptor class; the pattern of agonist potencies suggests that the receptor is very similar to the 5-HT1D receptor subtype.
The pharmacological properties of the presynaptic serotonin autoreceptor in the pig brain cortex conform to the 5-HT1D receptor subtype.[Pubmed:2797214]
Naunyn Schmiedebergs Arch Pharmacol. 1989 Jul;340(1):45-51.
The effects of serotonin receptor agonists and antagonists on the electrically (3 Hz) evoked 3H overflow were determined on pig brain cortex slices preincubated with 3H-serotonin and superfused with physiological salt solution containing indalpine (an inhibitor of serotonin uptake) plus phentolamine. The potencies of the serotonin receptor agonists and antagonists were compared with their affinities for 5-HT1A, 5-HT1B, 5-HT1C, and 5-HT1D binding sites in pig or rat tissue membranes; in addition, the potencies of the agonists were compared to their potencies in inhibiting adenylate cyclase activity in membranes of calf substantia nigra. In the superfusion experiments on pig brain cortex slices the following rank orders of potencies were obtained: agonists, serotonin greater than 5-methoxytryptamine = 5-carboxamidotryptamine greater than RU 24969 (5-methoxy-3(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole) greater than SDZ 21009 (4(3-terbutylamino-2-hydroxypropoxy)indol-2-carbonic-acid-isopr opylester) greater than or equal to yohimbine greater than or equal to cyanopindolol greater than 8-OH-DPAT (8-hydroxy-2-(di-n-propylamino)tetralin) greater than or equal to CGS 12066 B (7-trifluoromethyl-4(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]quinoxaline); ipsapirone and urapidil were ineffective; antagonists (antagonism determined against 5-methoxytryptamine as an agonist), metitepine greater than metergoline greater than mianserin. Propranolol, spiperone or mesulergine did not produce a shift of the concentration-response curve for 5-methoxytryptamine.(ABSTRACT TRUNCATED AT 250 WORDS)
International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin).[Pubmed:7938165]
Pharmacol Rev. 1994 Jun;46(2):157-203.
It is evident that in the last decade or so, a vast amount of new information has become available concerning the various 5-HT receptor types and their characteristics. This derives from two main research approaches, operational pharmacology, using selective ligands (both agonists and antagonists), and, more recently, molecular biology. Although the scientific community continues to deliberate about the hierarchy of criteria for neurotransmitter receptor characterisation, there seems good agreement between the two approaches regarding 5-HT receptor classification. In addition, the information regarding transduction mechanisms and second messengers is also entirely consistent. Thus, on the basis of these essential criteria for receptor characterisation and classification, there are at least three main groups or classes of 5-HT receptor: 5-HT1, 5-HT2, and 5-HT3. Each group is not only operationally but also structurally distinct, with each receptor group having its own distinct transducing system. The more recently identified 5-HT4 receptor almost undoubtedly represents a fourth 5-HT receptor class on the basis of operational and transductional data, but this will only be definitively shown when the cDNA for the receptor has been cloned and the amino acid sequence of the protein is known. Although those 5-HT receptors that have been fully characterised and classified to date (and, hence, named with confidence) would seem to mediate the majority of the actions of 5-HT throughout the mammalian body, not all receptors for 5-HT are fully encompassed within our scheme of classification. These apparent anomalies must be recognised and need further study. They may or may not represent new groups of 5-HT receptor or subtypes of already known groups of 5-HT receptor. Even though the cDNAs for the 5-ht1E, 5-ht1F, 5-ht5, 5-ht6, and 5-ht7 receptors have been cloned and their amino acid sequence defined, more data are necessary concerning their operational and transductional characteristics before one can be confident of the suitability of their appellations. Therefore, it is important to rationalise in concert all of the available data from studies involving both operational approaches of the classical pharmacological type and those from molecular and cellular biology.(ABSTRACT TRUNCATED AT 400 WORDS)


