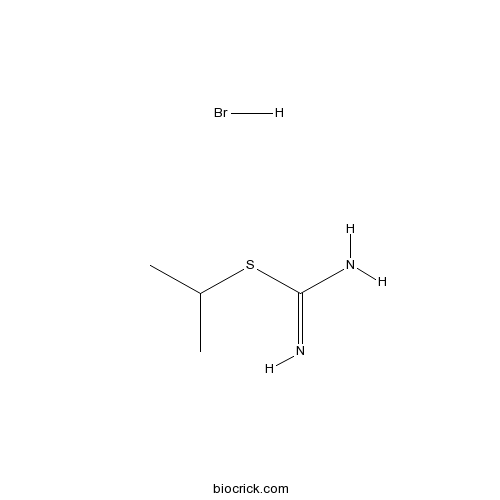
S-Isopropylisothiourea hydrobromideiNOS inhibitor, acts at arginine binding site CAS# 4269-97-0 |
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4269-97-0 | SDF | Download SDF |
| PubChem ID | 9813062 | Appearance | Powder |
| Formula | C4H11BrN2S | M.Wt | 199.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | propan-2-yl carbamimidothioate;hydrobromide | ||
| SMILES | CC(C)SC(=N)N.Br | ||
| Standard InChIKey | SLGVZEOMLCTKRK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H10N2S.BrH/c1-3(2)7-4(5)6;/h3H,1-2H3,(H3,5,6);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of NO synthase, with selectivity for the inducible form. |

S-Isopropylisothiourea hydrobromide Dilution Calculator

S-Isopropylisothiourea hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0223 mL | 25.1117 mL | 50.2235 mL | 100.447 mL | 125.5587 mL |
| 5 mM | 1.0045 mL | 5.0223 mL | 10.0447 mL | 20.0894 mL | 25.1117 mL |
| 10 mM | 0.5022 mL | 2.5112 mL | 5.0223 mL | 10.0447 mL | 12.5559 mL |
| 50 mM | 0.1004 mL | 0.5022 mL | 1.0045 mL | 2.0089 mL | 2.5112 mL |
| 100 mM | 0.0502 mL | 0.2511 mL | 0.5022 mL | 1.0045 mL | 1.2556 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NS 5806
Catalog No.:BCC7872
CAS No.:426834-69-7
- 4-Hydroxy-3-methoxyphenyl O-beta-D-6-O-syringate-glucopyranoside
Catalog No.:BCN1442
CAS No.:426821-85-4
- Hemapolin
Catalog No.:BCC8994
CAS No.:4267-80-5
- Dehydrodiconiferyl alcohol
Catalog No.:BCN6878
CAS No.:4263-87-0
- 21,23-Dihydro-23-hydroxy-21-oxozapoterin
Catalog No.:BCN7230
CAS No.:426266-88-8
- TAK-700 salt
Catalog No.:BCC1979
CAS No.:426219-53-6
- TAK-700 (Orteronel)
Catalog No.:BCC2280
CAS No.:426219-18-3
- Luteolin-6-C-glucoside
Catalog No.:BCN4985
CAS No.:4261-42-1
- 8-Hydroxy-5-O-beta-D-glucopyranosylpsoralen
Catalog No.:BCN1443
CAS No.:425680-98-4
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- Crocin
Catalog No.:BCN2373
CAS No.:42553-65-1
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Cyproterone Acetate
Catalog No.:BCC3758
CAS No.:427-51-0
- Pedunculoside
Catalog No.:BCN1191
CAS No.:42719-32-4
- 2-Aminophenyl phenyl sulfone
Catalog No.:BCC8554
CAS No.:4273-98-7
- D-Valinol
Catalog No.:BCC2693
CAS No.:4276-09-9
- Magnoflorine Iodide
Catalog No.:BCN2911
CAS No.:4277-43-4
- H-N-Me-Leu-OBzl.TosOH
Catalog No.:BCC3215
CAS No.:42807-66-9
- Shinjulactone L
Catalog No.:BCN7958
CAS No.:4283-49-2
- Catechin 7-xyloside
Catalog No.:BCN5484
CAS No.:42830-48-8
- Flumequine
Catalog No.:BCC5090
CAS No.:42835-25-6
- Flumequine sodium
Catalog No.:BCC8985
CAS No.:42835-68-7
- H-Ala-OBzl.TosOH
Catalog No.:BCC3191
CAS No.:42854-62-6
- 2-(3-Benzoylphenyl)propionitrile
Catalog No.:BCC8479
CAS No.:42872-30-0
A Sensitive Precolumn Derivatization Method for Determination of Piperazine in Vortioxetine Hydrobromide Using a C8 Column and High-Performance Liquid Chromatography-Mass Spectrometry.[Pubmed:27941264]
Anal Sci. 2016;32(12):1333-1338.
A rapid and sensitive high-performance liquid chromatography-mass spectrometry method was established to determine the trace residues of piperazine in vortioxetine hydrobromide. The presence of piperazine was determined by precolumn derivatization with dansyl chloride. Chromatographic separation was performed on a Waters SunFire C8 column (150 x 4.6 mm, 3.5 mum) in gradient elution mode, using formic acid and acetonitrile as mobile phase. Detection was performed in a single quadrupole mass spectrometer in single ion monitoring mode using positive ionization. An m/z value of 553 was selected for monitoring disubstituted piperazine by DNS-Cl. Linearity, accuracy, and precision were found to be acceptable over the piperazine concentration range of 0.3525 - 2.35 ng mL(-1). The limit of detection and limit of quantification of piperazine were 0.1175 and 0.3525 ng mL(-1), respectively, which complied with the requirements of qualitative and quantitative analyses. The method was deemed sensitive and efficient.
Pharmacologic Evaluation of Antidepressant Activity and Synthesis of 2-Morpholino-5-phenyl-6H-1,3,4-thiadiazine Hydrobromide.[Pubmed:27213404]
Pharmaceuticals (Basel). 2016 May 19;9(2). pii: ph9020027.
Substituted thiadiazines exert a reliable therapeutic effect in treating stress, and a schematic description of their ability to influence all aspects of a stress response has been depicted. This study was conducted to pharmacologically evaluate compound L-17, a substituted thiadiazine, (2-morpholino-5-phenyl-6H-1,3,4-thiadiazine, hydrobromide) for possible anti-psychotic/antidepressant activity. Compound L-17 was synthesized by cyclocondensation of alpha-bromoacetophenone with the original morpholine-4-carbothionic acid hydrazide. Pharmacologic evaluations were conducted using methods described by E.F. Lavretskaya (1985), and in accordance with published guidelines for studying drugs for neuroleptic activity. Compound L-17 was evaluated for various possible mechanisms of action, including its effects on cholinergic system agonists/antagonists, dopaminergic neurotransmission, the adrenergic system, and 5-HT3 serotonin receptors. One or more of these mechanisms may be responsible for the beneficial effects shown by thiadiazine compounds in experiments conducted to evaluate their activity in models of acute stress and acute myocardial infarction.
Glycopyrrolate in comparison to hyoscine hydrobromide and placebo in the treatment of hypersalivation induced by clozapine (GOTHIC1): study protocol for a randomised controlled feasibility study.[Pubmed:27871302]
Trials. 2016 Nov 21;17(1):553.
BACKGROUND: Clozapine is the only medication licensed for the treatment of resistant schizophrenia in the UK. Although efficacious, a common and unpopular side effect of clozapine treatment is clozapine-induced hypersalivation (CIH), which can contribute to non-adherence. The standard treatment for CIH in the UK is hyoscine hydrobromide but this may aggravate cognitive deficits in patients with schizophrenia while glycopyrrolate may be an effective alternative with a more tolerable side effect profile. There is currently no convincing evidence for hyoscine, or any other medication, as an effective treatment for CIH. METHODS/DESIGN: This is a multicentre randomised, double-blind, placebo-controlled feasibility study of glycopyrronium bromide (glycopyrrolate) and hyoscine hydrobromide (hyoscine) in patients with clozapine-induced hypersalivation. We aim to recruit 42 patients who have been prescribed clozapine and are experiencing hypersalivation, and randomise them to one of three study arms (either hyoscine, glycopyrrolate or placebo). The primary outcome measures will be the participant recruitment and attrition rates, and the secondary outcome will be the metrics of the daytime hypersalivation measure. After a 1-week washout period (discontinuing CIH medication, if any), there will be a 4-week treatment period where participants will be titrated up to the maximum tolerated dose of hyoscine, glycopyrrolate or placebo. Measurements of daytime salivation, nocturnal salivation, cognition and side effects will be taken during home visits in week 2 and week 5. Information on salivation and side effects will also be taken through telephone calls in week 3 and week 4. To gather information on the experience of study participants, exit interviews will also be requested with all participants who drop out of the study and a sample of participants who complete the study. DISCUSSION: There is currently no convincing evidence for hyoscine, or any other medication, as an effective treatment for CIH. There is promising evidence that glycopyrrolate may be more successful in the treatment of CIH causing fewer cognitive side effects. We propose to conduct a randomised placebo-controlled feasibility study of glycopyrrolate and hyoscine in the treatment of clozapine-induced hypersalivation to inform the design of a future efficacy trial. TRIAL REGISTRATION: Clinicaltrials.gov NCT02613494 , 23 November 2015.
[The Study of Evidence Base for the Use of Lappaconitine Hydrobromide in Patients With Atrial Fibrillation].[Pubmed:28294889]
Kardiologiia. 2016 Mar;56(3):48-53.
Lappaconitine Hydrobromide (LH, allapinin) has been included by authors of National Guidelines on Diagnosis and Treatment of Atrial Fibrillation (AF), 2012 in the number of medications recommended for use in patients with AF for rhythm control. Moreover, LH is also included into the List of Vital and Essential Medicinal Drugs (VEMD) 2015. However, LH is not mentioned in corresponding guidelines of the European Society of Cardiology (ESC). Aim of the present review was to explore evidence base underlining use of LH in the context of AF and to understand reason for LH-related discrepancy between European and domestic guidelines. RESULTS: Literature search has indicated that efficacy of LH was assessed only in small open studies. None of prospective trials included more than 100 patients. For more than 25 years of presence on the market slightly more than 400 patients were administered LH in clinical studies. In the only trial, designated as randomized number of participants (only men younger than 60 years) was small and the comparator was quinidine that presently is not used for maintenance of sinus rhythm in AF. Another study referenced in domestic guidelines on management of AF was observational and not intended for comparison of antiarrhythmic activity of drugs. CONCLUSION: Design of studies reviewed as well as their results provide insufficient evidence supporting the use of LH for maintenance of sinus rhythm in routine management of AF. At present inclusion of LH in guidelines on AF management and in the List of VEMD appears unjustified.
Selective inhibitors of neuronal nitric oxide synthase--is no NOS really good NOS for the nervous system?[Pubmed:9226999]
Trends Pharmacol Sci. 1997 Jun;18(6):204-11.
It is now ten years since NO was shown to account for the biological activity of endothelium-derived relaxing factor (EDRF). It is also the tenth anniversary of the identification of L-NG monomethyl arginine (L-NMMA) as the very first inhibitor of NO biosynthesis. That EDRF and NO were one and the same sparked an explosion of interest in the biochemistry and pharmacology of NO which has yet to subside. In contrast, the first ever nitric oxide synthase (NOS) inhibitor slipped seamlessly into the literature virtually without comment at the time. Over the following decade, L-NMMA (and like NOS inhibitors) have proved invaluable as tools for probing the biological roles of NO in health and disease and, in particular, have increased our understanding of the function of NO in the nervous system. Further advances in this important area now require the development of inhibitors selective for the neuronal isoform of NOS (nNOS). Here, Philip Moore and Rachel Handy provide an up-to-date account of the literature regarding the biochemical and pharmacological characterization of NOS inhibitors with particular reference to compounds with greater selectivity for the nNOS isoform.
Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas.[Pubmed:7523409]
J Biol Chem. 1994 Oct 28;269(43):26669-76.
S-Ethylisothiourea was a potent competitive inhibitor of human nitric oxide synthase (NOS), with Ki values of 17, 36, and 29 nM for the inducible (i), endothelial (e), and neuronal (n) isozymes, respectively. Unlike some potent inhibitors of NOS, no time dependence was observed. S-Ethylisothiourea was not a detectable substrate for eNOS. S-Ethylisothiourea was also a potent inhibitor of mouse iNOS (Ki value of 5.2 nM), and its binding perturbed the spectrum of iNOS consistent with its altering the environment of the bound heme. The optimum binding of S-ethyl- and S-isopropylisothiourea relative to 70 other analogs suggested that these alkyl substitutions fit into a small hydrophobic pocket. Most isothioureas were 2-6-fold selective for the human iNOS (Ki for iNOS versus Ki for eNOS), with one being 19-fold selective. The cyclized mimics of S-ethylisothiourea, 2-NH2-thiazoline, and 2-NH2-thiazole, were also competitive inhibitors of human NOS. A third structural class of inhibitors, bisisothioureas, were, in general, the most selective in their inhibition of human iNOS. S,S'-(1,3-Phenylenebis(1,2-ethanediyl))bisisothiourea was 190-fold selective (Ki value of 0.047 microM against iNOS versus 9.0 microM against eNOS). These results demonstrate that potent and selective inhibition of human NOS isozymes is achievable.


