RubiadinCAS# 117-02-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117-02-2 | SDF | Download SDF |
| PubChem ID | 124062 | Appearance | Yellow powder |
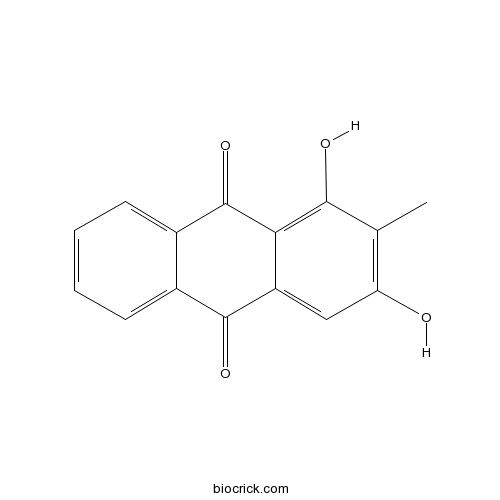
| Formula | C15H10O4 | M.Wt | 254.2 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Synonyms | 1,3-Dihydroxy 2-methylanthraquinone | ||
| Solubility | Soluble in acetonitrile, DMSO, ethanol and ethyl acetate | ||
| Chemical Name | 1,3-dihydroxy-2-methylanthracene-9,10-dione | ||
| SMILES | CC1=C(C=C2C(=C1O)C(=O)C3=CC=CC=C3C2=O)O | ||
| Standard InChIKey | IRZTUXPRIUZXMP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O4/c1-7-11(16)6-10-12(13(7)17)15(19)9-5-3-2-4-8(9)14(10)18/h2-6,16-17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rubiadin has hepatoprotective, antioxidant, and antitumor promoting effects. Rubiadin can decrease bone loss through the inhibition of osteoclast formation,differentiation and bone resorption. |
| Targets | Caspase | PARP |
| In vitro | Photochemotherapy using natural anthraquinones: Rubiadin and Soranjidiol sensitize human cancer cell to die by apoptosis.[Pubmed: 24561303]Photodiagnosis Photodyn Ther. 2014 Jun;11(2):182-92.Over the past decade the science has studied synthetic photosensitizers used in photodynamic therapy (PDT) or photochemotherapy as anticancer candidates. In this context, compounds extracted from vegetable species present interesting potential in the cancer field. Free radical scanvenging and antioxidant effects of some anthraquinone derivatives.[Pubmed: 22313303]Med Chem. 2011 Nov;7(6):639-44.
Antitumor promoting and actioxidant activities of anthraquinones isolated from the cell suspension culture of Morinda elliptica[Reference: WebLink]Asia-Pacific Journal of Molecular Biology and Biotechnology, 2003, 11(1):3-7.Six anthraquinones (nordamnacanthal, alizarin-1-methyl ether, Rubiadin, soranjidiol, lucidin-ω-methyl ether and morindone) isolated from the cell suspension culture of Morinda elliptica were assayed for antitumor promoting and antioxidant activities. |
| Kinase Assay | Effects of Rubiadin from Morinda officinalis on Osteoclastic Bone Resorption[Reference: WebLink]Pharmaceutical Journal of Chinese Peoples Liberation Army, 2009, 25(6):505-9.To investigate the cellular mechanism of Rubiadin inhibitory effects on bone resorption. |

Rubiadin Dilution Calculator

Rubiadin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9339 mL | 19.6696 mL | 39.3391 mL | 78.6782 mL | 98.3478 mL |
| 5 mM | 0.7868 mL | 3.9339 mL | 7.8678 mL | 15.7356 mL | 19.6696 mL |
| 10 mM | 0.3934 mL | 1.967 mL | 3.9339 mL | 7.8678 mL | 9.8348 mL |
| 50 mM | 0.0787 mL | 0.3934 mL | 0.7868 mL | 1.5736 mL | 1.967 mL |
| 100 mM | 0.0393 mL | 0.1967 mL | 0.3934 mL | 0.7868 mL | 0.9835 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sculponeatic acid
Catalog No.:BCN6046
CAS No.:1169806-02-3
- Sculponeatin O
Catalog No.:BCN6045
CAS No.:1169806-00-1
- Sculponeatin N
Catalog No.:BCN6044
CAS No.:1169805-98-4
- INDY
Catalog No.:BCC6349
CAS No.:1169755-45-6
- XL413 hydrochloride
Catalog No.:BCC4039
CAS No.:1169562-71-3
- XL413
Catalog No.:BCC4241
CAS No.:1169558-38-6
- Monohydroxyisoaflavinine
Catalog No.:BCN7284
CAS No.:116865-09-9
- 20-Hydroxyaflavinine
Catalog No.:BCN7283
CAS No.:116865-08-8
- Fmoc-D-Ser-OH
Catalog No.:BCC3547
CAS No.:116861-26-8
- 5-Formamide-1-(2-formyloxyethl)pyrazole
Catalog No.:BCC8747
CAS No.:116856-18-9
- GDC-0623
Catalog No.:BCC4150
CAS No.:1168091-68-6
- 4',5,6,7-Tetramethoxyflavone
Catalog No.:BCN8256
CAS No.:1168-42-9
- Dantron
Catalog No.:BCN6048
CAS No.:117-10-2
- Quercetin
Catalog No.:BCN6049
CAS No.:117-39-5
- 2-Amino-3-hydroxyanthraquinone
Catalog No.:BCC8527
CAS No.:117-77-1
- 2-Anthraquinonecarboxylic acid
Catalog No.:BCN3451
CAS No.:117-78-2
- Bis(2-ethylhexyl) phthalate
Catalog No.:BCN6054
CAS No.:117-81-7
- Boc-Asp(Ofm)-OH
Catalog No.:BCC3366
CAS No.:117014-32-1
- Anwuweizonic acid
Catalog No.:BCN3633
CAS No.:117020-59-4
- Licopyranocoumarin
Catalog No.:BCN7900
CAS No.:117038-80-9
- 3'-Methoxypuerarin
Catalog No.:BCN2900
CAS No.:117047-07-1
- Wulignan A1
Catalog No.:BCN5808
CAS No.:117047-76-4
- Combretastatin A4
Catalog No.:BCC7089
CAS No.:117048-59-6
- 3'-Hydroxypuerarin
Catalog No.:BCN2816
CAS No.:117060-54-5
Rubiadin, a new antioxidant from Rubia cordifolia.[Pubmed:9425750]
Indian J Biochem Biophys. 1997 Jun;34(3):302-6.
Rubiadin, a dihydroxy anthraquinone, isolated from alcoholic extract of Rubia cordifolia, possesses potent antioxidant property. It prevents lipid peroxidation induced by FeSO4 and t-butylhydroperoxide (t-BHP) in a dose dependent manner. The per cent inhibition was more in the case of Fe2+ induced lipid peroxidation. The antioxidant property of the preparation has been found to be better than that of EDTA, Tris, mannitol, Vitamin E and p-benzoquinone.
Free radical scanvenging and antioxidant effects of some anthraquinone derivatives.[Pubmed:22313303]
Med Chem. 2011 Nov;7(6):639-44.
In this study, the screening of five anthraquinones (purpurin, xanthopurpurin, Rubiadin, kermisic acid and flavokermisic acid), for their free radical scavenging and antioxidant effects was carried out, using three complementary methods. DPPH (2,2'-diphenyl-1-picrylhydrazyl) revealed that purpurin has a scavenging effect with IC50 = 3.491 +/- 0.014 microg/ml. Results of beta-carotene/linoleic acid assay showed that kermisic and flavokermisic acids have significant inhibition of lipid peroxidation with I % = 76.1 +/- 1.5% and 68.6 +/- 2.5%, respectively. In addition, the ferrous ion chelating test showed that only purpurin, with small concentrations, interferes in a dose dependant manner with the formation of Fe2+-ferrozine complex. These results are promising for further studies of the biological and pathological effects of these natural products.
Photochemotherapy using natural anthraquinones: Rubiadin and Soranjidiol sensitize human cancer cell to die by apoptosis.[Pubmed:24561303]
Photodiagnosis Photodyn Ther. 2014 Jun;11(2):182-92.
Over the past decade the science has studied synthetic photosensitizers used in photodynamic therapy (PDT) or photochemotherapy as anticancer candidates. In this context, compounds extracted from vegetable species present interesting potential in the cancer field. In our laboratory, we studied Heterophyllaea pustulata a phototoxic shrub that habit the northwest of Argentina. From this vegetal, by in vitro germination, we obtained Rubiadin and Soranjidiol, two anthraquinones that exhibited significant photocytotoxicity on human cancer cells. In addition, the fraction obtained from callus cultures allowed us to get a satisfactory content of these compounds compared to those found from the original plant. Under PDT regimen, we found that cell destruction resulted in a dose-dependent manner and occasioned apoptosis on photosensitized cells. Biochemical analysis revealed the involvement of caspase-3, PARP cleavage and DNA fragmentation in Rubiadin induced apoptosis. Moreover, Soranjidiol-PDT led to mu-calpain-induced apoptosis involving caspases-3-independent DNA fragmentation. We also showed that both anthraquinones are cytoplasmatically distributed and out of nucleus. In addition, we demonstrated a synergic cytotoxic effect when we combined them. Our data demonstrated that Rubiadin and Soranjidiol could be further considered as natural photocytotoxic compounds against cancer cells and callus cultures are a plausible source of these anthraquinonic compounds.


