RocaglaolCAS# 147059-46-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147059-46-9 | SDF | Download SDF |
| PubChem ID | 393602 | Appearance | Powder |
| Formula | C26H26O6 | M.Wt | 434.5 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
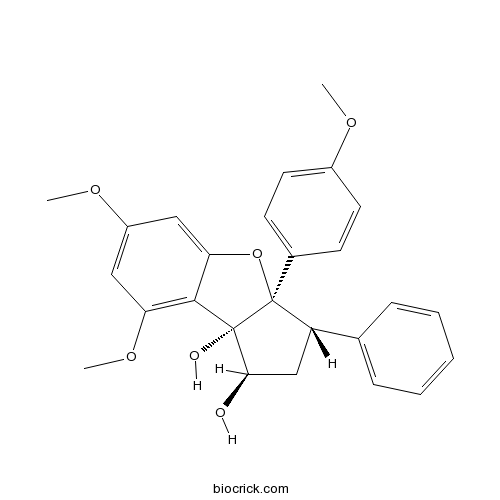
| Chemical Name | (1R,3S,3aR,8bS)-6,8-dimethoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3-dihydro-1H-cyclopenta[b][1]benzofuran-1,8b-diol | ||
| SMILES | COC1=CC=C(C=C1)C23C(CC(C2(C4=C(C=C(C=C4O3)OC)OC)O)O)C5=CC=CC=C5 | ||
| Standard InChIKey | RRVZOJQBRVGMMK-HCBGRYSISA-N | ||
| Standard InChI | InChI=1S/C26H26O6/c1-29-18-11-9-17(10-12-18)26-20(16-7-5-4-6-8-16)15-23(27)25(26,28)24-21(31-3)13-19(30-2)14-22(24)32-26/h4-14,20,23,27-28H,15H2,1-3H3/t20-,23+,25+,26-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Rocaglaol is a cytotoxic cyclopenta[b]benzofuran, shows in vitro cytotoxic activity against Lu1, LNCaP and MCF-7 cells with ED50 values of 13.8, 23.0 and 9.2 nM, respectively. 2. Rocaglaol is a potent anticancer drug that induces apoptosis of LNCaP cells through the mitochondrial pathway and its G2/M-phase cell cycle arrest is associated with the down-regulation of Cdc25C and the dephosphorylation of Cdc2. 3. Rocaglaol can reduce tissue inflammation and neuronal cell death by inhibiting NF-kappa B and AP-1 signaling, resulting in significant neuroprotection in animal models of neurodegeneration. 4. Rocaglaol derivatives can prevent or to limit the cardiotoxicity of an antineoplastic agent, in particular to prevent or to limit the apoptosis of cardiomyocytes induced by such agent. 5. Rocaglaol, pyrimidinone and aglaiastatin are potent inhibitors of the growth of K--cells, with IC50 values of 1-10 ng/mL, and induce normal morphology in K--cells at 10-30 ng/mL, they also specifically inhibit protein synthesis. |
| Targets | Bcl-2/Bax | TNF-α | AP-1 | NF-kB |

Rocaglaol Dilution Calculator

Rocaglaol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3015 mL | 11.5075 mL | 23.015 mL | 46.0299 mL | 57.5374 mL |
| 5 mM | 0.4603 mL | 2.3015 mL | 4.603 mL | 9.206 mL | 11.5075 mL |
| 10 mM | 0.2301 mL | 1.1507 mL | 2.3015 mL | 4.603 mL | 5.7537 mL |
| 50 mM | 0.046 mL | 0.2301 mL | 0.4603 mL | 0.9206 mL | 1.1507 mL |
| 100 mM | 0.023 mL | 0.1151 mL | 0.2301 mL | 0.4603 mL | 0.5754 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyclo(Phe-Pro)
Catalog No.:BCN2416
CAS No.:14705-60-3
- MK591
Catalog No.:BCC1766
CAS No.:147030-01-1
- Menthyl-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-[1,3]oxathiolane-2-carboxylic acid
Catalog No.:BCC9019
CAS No.:147027-10-9
- 3'-O-Demethylarctigenin
Catalog No.:BCN3544
CAS No.:147022-95-5
- Cytarabine
Catalog No.:BCC3759
CAS No.:147-94-4
- Proline
Catalog No.:BCN1656
CAS No.:147-85-3
- DL-Arabinose
Catalog No.:BCN8541
CAS No.:147-81-9
- Diphenhydramine hydrochloride
Catalog No.:BCC8947
CAS No.:147-24-0
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- Atglistatin
Catalog No.:BCC5104
CAS No.:1469924-27-3
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Y-27632
Catalog No.:BCC4301
CAS No.:146986-50-7
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
- Alcaftadine
Catalog No.:BCC5260
CAS No.:147084-10-4
- 5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one
Catalog No.:BCC8722
CAS No.:147086-79-1
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- Maropitant
Catalog No.:BCC1728
CAS No.:147116-67-4
- Methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
Catalog No.:BCC9031
CAS No.:147118-35-2
- 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl
Catalog No.:BCC8651
CAS No.:147118-37-4
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- 4-Chloro-L-phenylalanine Hydrochloride
Catalog No.:BCC2638
CAS No.:123053-23-6
- TT 232
Catalog No.:BCC6248
CAS No.:147159-51-1
- N-6-Methyl-7,7-dioxo-2-sulfamoyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-yl]acetamide
Catalog No.:BCC9077
CAS No.:147200-03-1
- Arecaidine propargyl ester tosylate
Catalog No.:BCC6628
CAS No.:147202-94-6
Rocaglaol induces apoptosis and cell cycle arrest in LNCaP cells.[Pubmed:16619491]
Anticancer Res. 2006 Mar-Apr;26(2A):947-52.
Rocaglaol is a cytotoxic cyclopenta[b]benzofuran isolated from the bark of Aglaia crassinervia. It exhibited in vitro cytotoxic activity against Lu1, LNCaP and MCF-7 cells with ED50 values of 13.8, 23.0 and 9.2 nM, respectively. DAPI staining indicated that LNCaP cells treated with Rocaglaol underwent apoptosis. In order to determine whether Rocaglaol-induced apoptosis is mediated by the mitochondrial pathway, apoptosis-related mitochondrial-associated proteins were studied. Rocaglaol treatment induced Bax expression through 12 to 72 h of exposure, while Bcl-xl expression was slightly decreased through 48 h, and decreased more significantly by 72 h. Cleaved caspase-9 expression was detected at 72 h, and cleaved caspase-7 was increased through 48 to 72 h. Consequently, the large fragment (89 kDa) of PARP resulting from caspase cleavage was detected at 12, 24 and 48 h, and especially at 72 h. Cleaved PARP expression was also detected at 72 h. Since Rocaglaol caused dose-dependent G2/M phase arrest of LNCaP cells as indicated by flow cytometric analysis, the protein levels of cell cycle-related genes were measured. Rocaglaol treatment (230 nM) did not change cyclin B after 24- to 60-h treatment. The expression of cdc2 was not changed and phospho-cdc2 (Tyr 15) increased after 36-, 48- and 60-h treatment. In addition, protein phosphatase Cdc25C, which functions as a mitotic activator by dephosphorylation of Cdc2, decreased in a time-dependent manner after Rocaglaol treatment. Taken together, these results suggest that Rocaglaol is a potent anticancer drug that induces apoptosis of LNCaP cells through the mitochondrial pathway and its G2/M-phase cell cycle arrest is associated with the down-regulation of Cdc25C and the dephosphorylation of Cdc2.
Cyclopentabenzofuran lignan protein synthesis inhibitors from Aglaia odorata.[Pubmed:8759160]
J Nat Prod. 1996 Jul;59(7):650-2.
In the course of screening for Ras function inhibitors, Rocaglaol (1) and the related compounds, the known pyrimidinone (2) and the novel aglaiastatin (3), were isolated from a CHCI3 extract of the leaves of Aglaia odorata. The structure of 3 was elucidated as a novel cyclopentabenzofuran on the basis of its NMR spectroscopic data and by X-ray crystallographic analysis. These compounds (1-3) were potent inhibitors of the growth of K-ras-NRK cells, with IC50 values of 1-10 ng/mL, and induced normal morphology in K-ras-NRK cells at 10-30 ng/mL. They also specifically inhibited protein synthesis. Aglaiastatin (3) was slightly more potent than 1 and 2 in inhibiting cell growth. Aglaiastatin (3) reduced the amount of Ras, possibly by inhibiting its de novo synthesis.
Synthetic analogue of rocaglaol displays a potent and selective cytotoxicity in cancer cells: involvement of apoptosis inducing factor and caspase-12.[Pubmed:19655762]
J Med Chem. 2009 Aug 27;52(16):5176-87.
Flavaglines constitute a family of natural anticancer compounds. We present here 3 (FL3), the first synthetic flavagline that inhibits cell proliferation and viability (IC(50) approximately 1 nM) at lower doses than did the parent compound, racemic Rocaglaol. Compound 3 enhanced doxorubicin cytotoxicity in HepG2 cells and retained its potency against adriamycin-resistant cell lines without inducing cardiomyocyte toxicity. Compound 3 induced apoptosis of HL60 and Hela cells by triggering the translocation of Apoptosis Inducing Factor (AIF) and caspase-12 to the nucleus. A fluorescent conjugate of 3 accumulated in endoplasmic reticulum (ER), suggesting that flavaglines bind to their target in the ER, where it triggers a cascade of events that leads to the translocation of AIF and caspase-12 to the nucleus and probably inhibition of eIF4A. Our studies highlight structural features critical to their antineoplastic potential and suggest that these compounds would retain their activity in cells refractory to caspase activation.
A synthetic derivative of the natural product rocaglaol is a potent inhibitor of cytokine-mediated signaling and shows neuroprotective activity in vitro and in animal models of Parkinson's disease and traumatic brain injury.[Pubmed:15716464]
Mol Pharmacol. 2005 May;67(5):1544-55.
Many acute and chronic neurodegenerative diseases are characterized by a localized inflammatory response and constitutive activation of the transcription factors nuclear factor-kappa B (NF-kappa B) and activator protein-1 (AP-1) as well as their upstream activating signaling cascades. Ample evidence indicates the implication of these processes in the pathogenesis of several diseases of the central nervous system. In this study, we show that a synthetic derivative of the natural product Rocaglaol (compound A) displays potent anti-inflammatory properties in human endothelial and murine glial cells in vitro. Compound A inhibited cytokine- and lipopolysaccharide-induced release of various cytokines/chemokines and of nitric oxide as well as expression of the adhesion molecule endothelial leukocyte adhesion molecule-1 and the inducible enzymes nitric-oxide synthase and cyclooxygenase-2. As shown by immunocytochemistry and immunoblotting, compound A inhibited NF-kappa B and AP-1 activity in mixed glial cultures. Compound A exhibited neuroprotective activity in vitro and in vivo. 1-Methyl-4-phenylpyridinium-induced damage of mesencephalic dopaminergic neurons was significantly decreased, and long-term treatment of 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine-injected mice with compound A significantly and dose-dependently reduced dopaminergic neuronal cell death. In addition, shortterm application of compound A to rats suffering from traumatic brain injury induced by subdural hematoma resulted in a significant reduction of the cerebral infarct volume. These results suggest that by inhibiting NF-kappa B and AP-1 signaling, compound A is able to reduce tissue inflammation and neuronal cell death, resulting in significant neuroprotection in animal models of neurodegeneration.


