RhusflavanoneCAS# 53060-72-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53060-72-3 | SDF | Download SDF |
| PubChem ID | 466314 | Appearance | Yellow powder |
| Formula | C30H22O10 | M.Wt | 542.5 |
| Type of Compound | Biflavanone | Storage | Desiccate at -20°C |
| Synonyms | Rhusflavone;Rhusflavanon | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
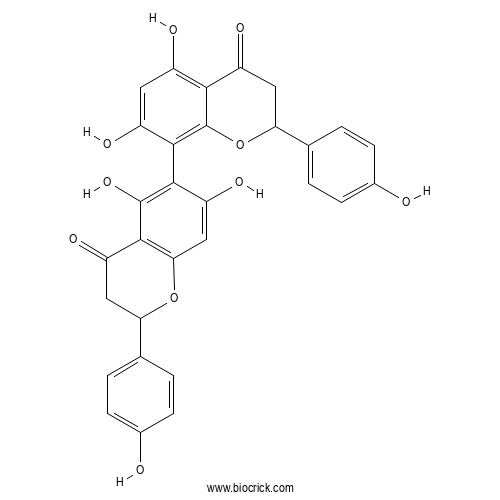
| Chemical Name | 6-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-2,3-dihydrochromen-8-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | C1C(OC2=C(C1=O)C(=C(C(=C2)O)C3=C4C(=C(C=C3O)O)C(=O)CC(O4)C5=CC=C(C=C5)O)O)C6=CC=C(C=C6)O | ||
| Standard InChIKey | YBDIZQWDBBOOFB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H22O10/c31-15-5-1-13(2-6-15)22-11-20(36)26-24(39-22)12-21(37)27(29(26)38)28-18(34)9-17(33)25-19(35)10-23(40-30(25)28)14-3-7-16(32)8-4-14/h1-9,12,22-23,31-34,37-38H,10-11H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Rhusflavanone Dilution Calculator

Rhusflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8433 mL | 9.2166 mL | 18.4332 mL | 36.8664 mL | 46.0829 mL |
| 5 mM | 0.3687 mL | 1.8433 mL | 3.6866 mL | 7.3733 mL | 9.2166 mL |
| 10 mM | 0.1843 mL | 0.9217 mL | 1.8433 mL | 3.6866 mL | 4.6083 mL |
| 50 mM | 0.0369 mL | 0.1843 mL | 0.3687 mL | 0.7373 mL | 0.9217 mL |
| 100 mM | 0.0184 mL | 0.0922 mL | 0.1843 mL | 0.3687 mL | 0.4608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Agathisflavone
Catalog No.:BCN0810
CAS No.:28441-98-7
- Roburin E
Catalog No.:BCN0809
CAS No.:137343-79-4
- Roburin D
Catalog No.:BCN0808
CAS No.:136199-93-4
- Roburin C
Catalog No.:BCN0807
CAS No.:137490-45-0
- Roburin B
Catalog No.:BCN0806
CAS No.:137370-65-1
- Roburin A
Catalog No.:BCN0805
CAS No.:132864-75-6
- Gambogoic acid A
Catalog No.:BCN0804
CAS No.:8879-23-49-1
- Moreollic acid
Catalog No.:BCN0803
CAS No.:173792-68-2
- Epigambogellic acid
Catalog No.:BCN0802
CAS No.:1352191-85-5
- Cannabisin M
Catalog No.:BCN0801
CAS No.:1831134-13-4
- 11beta,13-Dihydrolactucin
Catalog No.:BCN0800
CAS No.:83117-63-9
- 2-Phenylethyl-beta-glucopyranoside
Catalog No.:BCN0799
CAS No.:18997-54-1
- Elgonica dimer A
Catalog No.:BCN0813
CAS No.:132210-48-1
- 4-Methoxybenzoylacetic acid
Catalog No.:BCN0814
CAS No.:13422-77-0
- Cajanine
Catalog No.:BCN0815
CAS No.:87402-84-4
- Senkyunolide N
Catalog No.:BCN0816
CAS No.:140694-58-2
- Dehydrodicatechin A
Catalog No.:BCN0817
CAS No.:36048-23-4
- Barbaloin-related compound A
Catalog No.:BCN0818
CAS No.:473225-21-7
- Barbaloin-related compound B
Catalog No.:BCN0819
CAS No.:473225-22-8
- 3beta-(alpha-L-Arabinopyranosyloxy)urs-12,18-dien-28-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN0820
CAS No.:435269-07-1
- 3beta-Hydroxyurs-12,18-dien-28-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN0821
CAS No.:434942-42-4
- 10-Methoxygambogenic acid
Catalog No.:BCN0822
CAS No.:2095102-72-8
- 10-Hydroxyaloin B
Catalog No.:BCN0823
CAS No.:134863-92-6
- Gambogic acid A
Catalog No.:BCN0824
CAS No.:1592842-93-7
In vitro testing and computational analysis of specific phytochemicals with antiviral activities considering their possible applications against COVID-19.[Pubmed:35165493]
S Afr J Bot. 2022 Feb 10. pii: S0254-6299(22)00056-4.
The purpose of this study was to investigate the reservoir of natural products against the SARS-CoV-2 virus and to identify suitable candidates in order to recommend appropriate phytotherapy. Adequately prepared 65 molecules from traditional Chinese medicine with proven antiviral properties were subjected to docking analysis using AutoDock Vina 4 software with the aim to investigate binding affinity and interactions of compounds with Mpro from the SARS-CoV-2 virus. Biflavonoids and tannins show best docking scores with -9,80kcal/mol for biflavonoids and -9,00 kcal/mol for tannins. Biflavonoids: amentoflavone, agathistaflavone, robustaflavone, hinokiflavone and Rhusflavanone were tested for their radical scavenging activity. Partition coefficients were examined by RP-HPLC. Evaluation of drug-likeness properties of investigated biflavonoids suggested Rhusflavanone as a molecule with the best ADMET characteristics. Anti-inflammatory activity of Rhusflavanone was investigated in LPS stimulated RAW264.7 macrophages. Tested biflavonoids exibit beneficial effects against inflammation by scavenging free radicals and by suppressing the production of proinflammatory mediators by macrophages. Both predictions of affinity spectra for substances (PASS) and in vitro testing showed promising biological activity of investigated biflavonoids. A Quantum chemical study was performed in order to calculate the thermodynamic, molecular orbital, and electrostatic potential of selected molecules and to compare their biological and chemical features. Our results highlighted antioxidant, anti-inflammatory and antiviral properties of investigated compounds, emphasizing the significance of biflavonoid moiety to selected characteristics, which encourage further investigational strategies against COVID-19.
Anti-virulence activities of biflavonoids from Mesua ferrea L. flower.[Pubmed:31534074]
Drug Discov Ther. 2019;13(4):222-227.
Based on the anti-virulence activity on Salmonella, the ethyl acetate extract (EAE) of Mesua ferrea flower was investigated for its chemical constituents. Ten purified compounds were identified and assayed for their inhibitory activity against Type III secretion system (T3SS) by polyacrylamide gel electrophoresis (SDS-PAGE) and Western blots experiments. We found the biflavonoids, Rhusflavanone and mesuaferrone B, exhibited inhibitory effects on the secretion of Salmonella pathogenicity island 1 (SPI-1) effector proteins (SipA, B, C and D) without effecting the bacterial growth. In addition, 5, 6, 6'-trihydroxy-[1,1'-biphenyl]-3,3'-dicarboxylic acid (6) is a new natural product from M. ferrea flower.
Rhusflavanone and mesuaferrone B: tyrosinase and elastase inhibitory biflavonoids extracted from the stamens of Mesua ferrea L.[Pubmed:31135222]
Nat Prod Res. 2021 Mar;35(6):1024-1028.
Chemical isolation and bioactivity studies were conducted on the stamens of Mesua ferrea L., which are being used in a traditional skincare formulation in Myanmar. Rhusflavanone and mesuaferrone B were obtained as the main biflavonoids together with lupeol, five common flavonoids, and five phenolic compounds. After being identified by NMR and other spectroscopic analyses, these compounds were evaluated for their 1,1-diphenyl-2-picrylhydrazyl (DPPH)-radical scavenging, human leukocyte elastase inhibitory, and mushroom tyrosinase inhibitory activities. The two biflavonoids exhibited strong inhibitory activities against elastase and tyrosinase, but low DPPH-radical scavenging activities. The contents of Rhusflavanone and mesuaferrone B in the stamens were 0.35 +/- 0.04% and 0.55 +/- 0.06%, respectively. Moreover, lupeol was considered to be a cosmetically important component of the stamens because of its high content and strong elastase inhibitory activity. Rhusflavanone was reported to be isolated from M. ferrea for the first time.
Antiviral activities of biflavonoids.[Pubmed:10193201]
Planta Med. 1999 Mar;65(2):120-5.
Biflavonoids such as amentoflavone (1), agathisflavone (2), robustaflavone (3), hinokiflavone (4), volkensiflavone (5), Rhusflavanone (7), succedaneflavanone (9), all isolated from Rhus succedanea and Garcinia multiflora, as well as their methyl ethers and acetates, volkensiflavone hexamethyl ether (6), Rhusflavanone hexaacetate (8), and succedaneflavanone hexaacetate (10) were evaluated for their antiviral activities. The inhibitory activities against a number of viruses including respiratory viruses (influenza A, influenza B, respiratory syncytial, parainfluenza type 3, adenovirus type 5, and measles) and herpes viruses (HSV-1, HSV-2, HCMV, and VZV) were investigated. The results indicated that robustaflavone exhibited strong inhibitory effects against influenza A and influenza B viruses with EC50 values of 2.0 micrograms/ml and 0.2 microgram/ml, respectively, and selectivity index values (SI) of 16 and 454, respectively. Amentoflavone and agathisflavone also demonstrated significant activity against influenza A and B viruses. Amentoflavone and robustaflavone exhibited moderate anti-HSV-1 anti-HSV-2 activities with EC50 values of 17.9 micrograms/ml (HSV-1) and 48.0 micrograms/ml (HSV-2) and SI values of > 5.6 (HSV-1) and > 2.1 (HSV-2) for amentoflavone; EC50 values of 8.6 micrograms/ml (HSV-1) and 8.5 micrograms/ml (HSV-2), and SI values of > 11.6 (HSV-1) and > 11.8 (HSV-2) for robustaflavone. Rhusflavanone demonstrated inhibitory activities against influenza B, measles, and HSV-2 viruses with SI values of 9.3, 8 and > 6.4, respectively. Succedaneaflavanone exhibited inhibitory activities against influenza B virus and VZV with SI values of 15 and < 3.0, respectively.
In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora.[Pubmed:9322359]
J Nat Prod. 1997 Sep;60(9):884-8.
Eleven biflavonoids, including amentoflavone (1), agathisflavone (2), robustaflavone (3), hinokiflavone (4), volkensiflavone (5), morelloflavone (7), Rhusflavanone (9), succedaneaflavanone (10), GB-1a (11), GB-1a 7"-O-beta-glucoside (13), and GB-2a (14) isolated from Rhus succedanea and Garcinia multiflora, as well as their methyl ethers, volkensiflavone hexamethyl ether (6), morelloflavone heptamethyl ether (8), and GB-1a hexamethyl ether (12), were evaluated for their anti-HIV-1 RT activity. The results indicated that compounds 3 and 4 demonstrated similar activity against HIV-1 reverse transcriptase (RT), with IC50 values of 65 microM. Compounds 1, 2, 7, 11, and 14 were moderately active against HIV-1 RT, with IC50 values of 119 microM, 100 microM, 116 microM, 236 microM, and 170 microM, respectively. Morelloflavone (7) also demonstrated significant antiviral activity against HIV-1 (strain LAV-1) in phytohemagglutinin-stimulated primary human peripheral blood mononuclear cells at an EC50 value of 6.9 microM and a selectivity index value of approximately 10. The other biflavonoids were either weakly active, inactive, or not selective against HIV-1 in human lymphocytes.
Hinokiflavone, a cytotoxic principle from Rhus succedanea and the cytotoxicity of the related biflavonoids.[Pubmed:2526343]
Planta Med. 1989 Apr;55(2):166-8.
Hinokiflavone (1) was isolated as the cytotoxic principle from the drupes of Rhus succedanea L. A comparison of the cytotoxicity of 1 and other related biflavonoids, including amentoflavone (2), robustaflavone (3), agathisflavone (4), rhusflavone (5), Rhusflavanone (6) and its hexaacetate (7), succedaneaflavanone (8) and its hexaacetate (9), cupressuflavone (10), neoRhusflavanone (11), volkensiflavone (12) and its hexamethyl ether (13), spicataside (14) and its nonaacetate (15), morelloflavone (16) and its heptaacetate (17) and heptamethyl ether (18), GB-1a (19) and its hexamethyl ether (20) and 7"-O-beta-glucoside (21), and GB-2a (22), indicates that an ether linkage between two units of apigenin as seen in 1 is structurally required for significant cytotoxicity. Compounds 13 and 20 also demonstrated significant cytotoxicity.


