RhamnocitrinCAS# 569-92-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 569-92-6 | SDF | Download SDF |
| PubChem ID | 5320946 | Appearance | Yellow powder |
| Formula | C16H12O6 | M.Wt | 300.27 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Kaempferol 7-methyl ether; 7-Methylkaempferol; 3,4',5-Trihydroxy 7-methoxyflavone | ||
| Solubility | Soluble in methan | ||
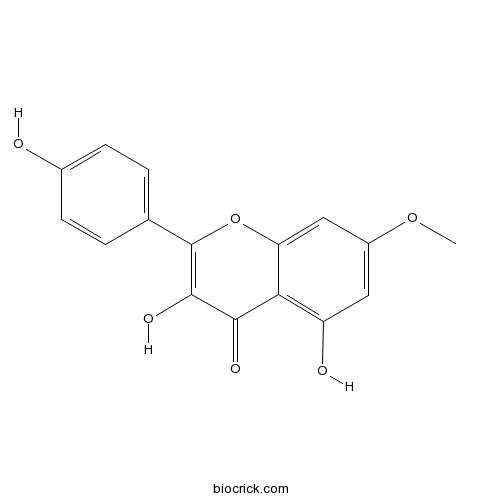
| Chemical Name | 3,5-dihydroxy-2-(4-hydroxyphenyl)-7-methoxychromen-4-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC(=C(C2=O)O)C3=CC=C(C=C3)O)O | ||
| Standard InChIKey | MQSZRBPYXNEFHF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O6/c1-21-10-6-11(18)13-12(7-10)22-16(15(20)14(13)19)8-2-4-9(17)5-3-8/h2-7,17-18,20H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Rhamnocitrin and kaempferol can augment cellular antioxidant defense capacity, at least in part, through regulation of HO-1 expression and MAPK signal transduction. 2. Rhamnocitrin and kaempferol not only protect low-density lipoprotein from oxidation but also prevent atherogenesis through suppressing macrophage uptake of oxidized low-density lipoprotein. 3. Rhamnocitrin can enhance the immune function, improve the formation of spleen cells of mice serum hemolysin of chicken red blood cell immune. 4. Rhamnocitrin shows a significant protection against cloudiness in lenses induced by hydrogen peroxide and hydrocortisone in a dose dependent manner, suggests that rhamnocitrin possesses significant anticataract activity and acts most likely due to its antioxidant property. |
| Targets | HO-1 | ERK | MEK | p38MAPK | Immunology & Inflammation related |

Rhamnocitrin Dilution Calculator

Rhamnocitrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3303 mL | 16.6517 mL | 33.3034 mL | 66.6067 mL | 83.2584 mL |
| 5 mM | 0.6661 mL | 3.3303 mL | 6.6607 mL | 13.3213 mL | 16.6517 mL |
| 10 mM | 0.333 mL | 1.6652 mL | 3.3303 mL | 6.6607 mL | 8.3258 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.6661 mL | 1.3321 mL | 1.6652 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.6661 mL | 0.8326 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nepetin-7-glucoside
Catalog No.:BCN2580
CAS No.:569-90-4
- Xanthohumol
Catalog No.:BCN5768
CAS No.:569-83-5
- Penduletin
Catalog No.:BCN5767
CAS No.:569-80-2
- Chlorotrianisene
Catalog No.:BCC6442
CAS No.:569-57-3
- Carcinine ditrifluoroacetate
Catalog No.:BCC7291
CAS No.:56897-53-1
- 8-Demethyleucalyptin
Catalog No.:BCN5765
CAS No.:5689-38-3
- Hemopressin (rat)
Catalog No.:BCC5807
CAS No.:568588-77-2
- Z-Glu(OBzl)-OH
Catalog No.:BCC2777
CAS No.:5680-86-4
- H-Ser-OMe.HCl
Catalog No.:BCC3029
CAS No.:5680-80-8
- H-Gly-OMe.HCl
Catalog No.:BCC2951
CAS No.:5680-79-5
- Tanshinone I
Catalog No.:BCN5764
CAS No.:568-73-0
- Tanshinone IIA
Catalog No.:BCN5763
CAS No.:568-72-9
- Splitomicin
Catalog No.:BCC3652
CAS No.:5690-03-9
- Boc-Ser(Tos)-OMe
Catalog No.:BCC3446
CAS No.:56926-94-4
- UBP 301
Catalog No.:BCC7172
CAS No.:569371-10-4
- 2'-O-Galloylmyricitrin
Catalog No.:BCN8252
CAS No.:56939-52-7
- Withanolide B
Catalog No.:BCN8011
CAS No.:56973-41-2
- Alnusdiol
Catalog No.:BCN6503
CAS No.:56973-51-4
- Platyphyllenone
Catalog No.:BCN5766
CAS No.:56973-65-0
- 9,9'-Di-O-(E)-feruloylsecoisolariciresinol
Catalog No.:BCN1415
CAS No.:56973-66-1
- Gabexate mesylate
Catalog No.:BCC2096
CAS No.:56974-61-9
- Boc-Cys(tBu)-OH
Catalog No.:BCC3379
CAS No.:56976-06-8
- U 46619
Catalog No.:BCC7207
CAS No.:56985-40-1
- NSC 87877
Catalog No.:BCC2468
CAS No.:56990-57-9
Regulation of heme oxygenase-1 expression and MAPK pathways in response to kaempferol and rhamnocitrin in PC12 cells.[Pubmed:19265714]
Toxicol Appl Pharmacol. 2009 May 15;237(1):59-68.
Oxidative stress has been considered as a major cause of cellular injuries in a variety of clinical abnormalities, especially neural diseases. Our aim of research is to investigate the protective effects and mechanisms of kaempferol and Rhamnocitrin (kaempferol-7-methyl ether) on oxidative damage in rat pheochromocytoma PC12 cells induced by a limited supply of serum and hydrogen peroxide (H2O2). The current result demonstrated that kaempferol protected PC12 cells from serum deprivation-induced apoptosis. Pretreatment of cells with kaempferol also diminished intracellular generation of reactive oxygen species (ROS) in response to H2O2 and strongly elevated cell viability. RT-Q-PCR and Western blotting revealed that kaempferol and Rhamnocitrin significantly induced heme oxygenase (HO)-1 gene expression. Addition of zinc protoporphyrin (Znpp), a HO-1 competitive inhibitor, significantly attenuated their protective effects in H2O2-treated cells, indicating the vital role of HO-1 in cell resistance to oxidative injury. While investigating the signaling pathways responsible for HO-1 induction, we observed that kaempferol induced sustained extracellular signal-regulated protein kinase 1/2 (ERK1/2) in PC12 cells grown in low serum medium; while Rhamnocitrin only stimulated transient ERK cascade. Addition of U0126, a highly selective inhibitor of MEK1/2, which is upstream of ERK1/2, had no effect on kaempferol- or Rhamnocitrin-induced HO-1 mRNA expression, indicating no direct cross-talk between these two pathways. Furthermore, both kaempferol and Rhamnocitrin were able to persistently attenuate p38 phosphorylation. Taking together, the above findings suggest that kaempferol and Rhamnocitrin can augment cellular antioxidant defense capacity, at least in part, through regulation of HO-1 expression and MAPK signal transduction.
Antiatherogenic effects of kaempferol and rhamnocitrin.[Pubmed:17973448]
J Agric Food Chem. 2007 Nov 28;55(24):9969-76.
Atherosclerosis is a chronic inflammatory disease of the arterial wall. Kaempferol and Rhamnocitrin (kaempferol 7-O-methyl ether) are two anti-inflammatory flavonoids commonly found in plants. The aim of this study is to investigate the function of kaempferol and Rhamnocitrin on prevention of atherosclerosis. Chemical analyses demonstrated that kaempferol and Rhamnocitrin were scavengers of DPPH (1,1-diphenyl-2-picrylhydrazyl) with IC50 of 26.10 +/- 1.33 and 28.38 +/- 3.07 microM, respectively. Copper-induced low-density lipoprotein (LDL) oxidation was inhibited by kaempferol and Rhamnocitrin, with similar potency, as measured by decreased formation of malondialdehyde and relative electrophoretic mobility (REM) on agarose gel, while Rhamnocitrin reduced delayed formation of conjugated dienes better than kaempferol. Cholesterol-laden macrophages are the hallmark of atherogenesis. The class B scavenger receptor, CD36, binds oxidized low-density lipoprotein (oxLDL), is found in atherosclerotic lesions, and is up-regulated by oxLDL. Addition of kaempferol and Rhamnocitrin (20 microM) caused significant reductions in cell surface CD36 protein expression in THP-1-derived macrophages (p < 0.05). Reverse transcription quantitative PCR (RT-Q-PCR) showed that kaempferol and Rhamnocitrin (20 microM) decreased oxLDL-induced CD36 mRNA expression (p < 0.01 and p < 0.05, respectively). Kaempferol- and Rhamnocitrin-treated macrophages also showed reduction in 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanide perchlorate (DiI)-labeled oxLDL uptake. Current evidences indicate that kaempferol and Rhamnocitrin not only protect LDL from oxidation but also prevent atherogenesis through suppressing macrophage uptake of oxLDL.


