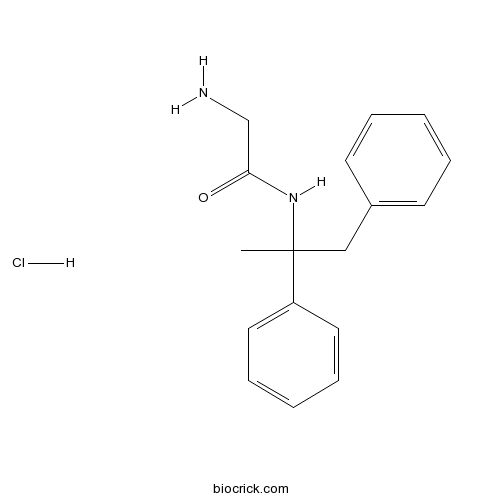
Remacemide hydrochlorideCAS# 111686-79-4 |
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- Pazopanib Hydrochloride
Catalog No.:BCC3708
CAS No.:635702-64-6
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 111686-79-4 | SDF | Download SDF |
| PubChem ID | 60510 | Appearance | Powder |
| Formula | C17H21ClN2O | M.Wt | 304.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | FPL 12924AA | ||
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | 2-amino-N-(1,2-diphenylpropan-2-yl)acetamide;hydrochloride | ||
| SMILES | CC(CC1=CC=CC=C1)(C2=CC=CC=C2)NC(=O)CN.Cl | ||
| Standard InChIKey | HYQMIUSWZXGTCC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H20N2O.ClH/c1-17(19-16(20)13-18,15-10-6-3-7-11-15)12-14-8-4-2-5-9-14;/h2-11H,12-13,18H2,1H3,(H,19,20);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-competitive NMDA receptor antagonist; blocks ion channel and allosteric modulatory site (IC50 = 8 - 68 mM). Anticonvulsant in vivo and metabolizes to a more potent desglycine analog. Weakly blocks voltage-dependent Na+ channels (IC50 = 161 mM). |

Remacemide hydrochloride Dilution Calculator

Remacemide hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2806 mL | 16.4031 mL | 32.8062 mL | 65.6125 mL | 82.0156 mL |
| 5 mM | 0.6561 mL | 3.2806 mL | 6.5612 mL | 13.1225 mL | 16.4031 mL |
| 10 mM | 0.3281 mL | 1.6403 mL | 3.2806 mL | 6.5612 mL | 8.2016 mL |
| 50 mM | 0.0656 mL | 0.3281 mL | 0.6561 mL | 1.3122 mL | 1.6403 mL |
| 100 mM | 0.0328 mL | 0.164 mL | 0.3281 mL | 0.6561 mL | 0.8202 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Elastase Inhibitor
Catalog No.:BCC1225
CAS No.:111682-13-4
- GSK1838705A
Catalog No.:BCC3787
CAS No.:1116235-97-2
- Cyanidin-3-O-arabinoside chloride
Catalog No.:BCN3023
CAS No.:111613-04-8
- Anonamine
Catalog No.:BCN2139
CAS No.:111566-66-6
- 3',5-Dihydroxy-4',5',6,7-tetramethoxyflavone
Catalog No.:BCN1620
CAS No.:111537-41-8
- Fmoc-D-Phg-OH
Catalog No.:BCC3316
CAS No.:111524-95-9
- Nyasicol
Catalog No.:BCN5999
CAS No.:111518-95-7
- Nyasicoside
Catalog No.:BCN5998
CAS No.:111518-94-6
- Metformin HCl
Catalog No.:BCC4799
CAS No.:1115-70-4
- L-Cysteinesulfinic acid
Catalog No.:BCC6571
CAS No.:1115-65-7
- H-Ala-OEt.HCl
Catalog No.:BCC2687
CAS No.:1115-59-9
- Ac-DL-Met-OH
Catalog No.:BCC2999
CAS No.:1115-47-5
- Demethoxyfumitremorgin C
Catalog No.:BCN7240
CAS No.:111768-16-2
- H-Glu-Oet.HCl
Catalog No.:BCC2685
CAS No.:1118-89-4
- (S)-(-)-HA-966
Catalog No.:BCC6589
CAS No.:111821-58-0
- 10-O-Methylprotosappanin B
Catalog No.:BCN6599
CAS No.:111830-77-4
- Hancinone C
Catalog No.:BCN4751
CAS No.:111843-10-8
- UCPH 101
Catalog No.:BCC7692
CAS No.:1118460-77-7
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
Influence of cytochrome P450 induction on the pharmacokinetics and pharmacodynamics of remacemide hydrochloride.[Pubmed:12076846]
Epilepsy Res. 2002 May;49(3):247-54.
Remacemide hydrochloride (RMD) is a putative anticonvulsant agent with an active metabolite, desglycinyl-remacemide (DGR) and a broad spectrum of activity in experimental seizure models. In clinical trials, however, the efficacy of RMD is questionable. In the case of add-on studies, the inconclusive findings may be related to pharmacokinetic interactions between RMD and established antiepileptic drugs. We have investigated the influence of cytochrome P450 (CYP(450)) induction following repeated treatment with phenobarbital (PB) on the pharmacokinetics and pharmacodynamics of RMD in mice. Pre-treatment with PB (80 mg/kg; once daily for 4 days) significantly increased CYP(450) content and activity in mouse liver. This was associated with a consistent reduction in the brain concentrations of both RMD and DGR and attenuation of the anticonvulsant effects of RMD in the maximal electroshock model. Pharmacokinetic analysis suggested that DGR was proportionately more susceptible to CYP(450) induction than the parent compound. As the principal active moiety, the selectively enhanced metabolism of DGR under induced conditions may underlie the debatable findings of add-on trials with RMD in refractory epilepsy. However, this hypothesis does not explain the similarly questionable efficacy of RMD monotherapy in newly diagnosed epilepsy, an observation that may have wider pharmacological implications.
Remacemide hydrochloride as an add-on therapy in epilepsy: a randomized, placebo-controlled trial of three dose levels (300, 600 and 1200 mg/day) in a Q.I.D. regimen.[Pubmed:11945098]
Seizure. 2002 Mar;11(2):114-23.
Remacemide hydrochloride is a low-affinity, non-competitive N-methyl-D-aspartic acid (NMDA) receptor channel blocker, under investigation in epilepsy. This double-blind, placebo-controlled, multicentre study assessed the safety and efficacy of Remacemide hydrochloride or placebo, as adjunctive therapy, in 252 adult patients with refractory epilepsy who were already taking up to three antiepileptic drugs (including an enzyme-inducer). Patients were randomized to one of three doses of Remacemide hydrochloride (300, 600 or 1200 mg /day) or placebo Q.I.D., for up to 15 weeks. An increasing percentage of responders (defined as a reduction in seizure frequency from baseline of > or =50%) was seen with increasing Remacemide hydrochloride dose. At 1200 mg /day, 23% of patients were responders compared with 7% on placebo. This difference was significant (P = 0.016), as was the overall difference between treatments (P = 0.038). Adverse events: dizziness, abnormal gait, gastrointestinal disturbance, somnolence, diplopia and fatigue were mild or moderate in severity. Carbamazepine and phenytoin plasma concentrations were well controlled and maintained within target ranges, with no evidence of improved seizure control due to increases in the concentrations of these drugs. A dose-dependent, significant, increase in responders following adjunctive Remacemide hydrochloride compared with placebo was observed. Remacemide hydrochloride was well tolerated.
A double-blind, placebo-controlled study of remacemide hydrochloride in patients with refractory epilepsy following pre-surgical assessment.[Pubmed:12160664]
Seizure. 2002 Sep;11(6):371-6.
This multicentre, randomised, double-blind, placebo-controlled, parallel-group study investigated the efficacy, safety and pharmacokinetics of Remacemide hydrochloride in adult patients ( n= 59) with refractory epilepsy, undergoing reduced or discontinued antiepileptic drug (AED) usage, as part of an evaluation for epilepsy surgery. On discontinuation or reduction of maintenance AEDs, patients received Remacemide hydrochloride, up to 600 mg daily, or placebo, for up to ten days or until they experienced a fourth complex partial (CPS) or a generalised tonic-clonic (GTC) seizure. Pre- and post-study blood and urine samples were taken for analysis. Remacemide hydrochloride showed a significantly ( P= 0.045) longer median time to fourth seizure compared with placebo (6.8 vs. 3.8 days). Median nine-day seizure counts were significantly ( P= 0.0327) lower with Remacemide hydrochloride than placebo (6.2 vs. 12.8). Eleven Remacemide hydrochloride patients and six placebo patients completed ten days' treatment. Remacemide and desglycinyl metabolite levels were lower in patients receiving concomitant carbamazepine or phenytoin than in those receiving non-inducing AEDs or Remacemide hydrochloride alone. No serious adverse events occurred; all patients receiving Remacemide hydrochloride completed the study. Remacemide hydrochloride was well tolerated and showed significant therapeutic activity in this patient population.
Remacemide hydrochloride as an add-on therapy in epilepsy: a randomized, placebo-controlled trial of three dose levels (300, 600 and 800 mg/day) in a B.I.D. regimen.[Pubmed:11945097]
Seizure. 2002 Mar;11(2):104-13.
Remacemide hydrochloride is a low-affinity, non-competitive NMDA receptor channel blocker under investigation for the treatment of epilepsy. This double-blind, placebo-controlled, multicentre study assessed the safety and efficacy of adjunctive Remacemide hydrochloride or placebo, in adult patients with refractory epilepsy who were already taking up to three antiepileptic drugs (including an enzyme-inducer). Patients (n= 262) were randomized to one of three doses of Remacemide hydrochloride (300, 600 or 800 mg/day) or placebo, in a B.I.D. regimen, for up to 14 weeks. Plasma concentrations of carbamazepine (CBZ) and phenytoin (PHT) were controlled throughout. Patients recorded their seizures on a diary card. There was an increase in the percentage of responders (defined as a reduction in seizure frequency from baseline > or = 50 %), from 15 % (9/60) with placebo, to 30 % (18/60) in the 800 mg/day group. A pairwise comparison between Remacemide hydrochloride 800 mg/day and placebo was statistically significant (P = 0.049). Most reported adverse events (mainly CNS and gastrointestinal) were mild or moderate in severity and dose-dependent. Adjunctive Remacemide hydrochloride treatment was associated with a higher, dose-related responder rate compared with placebo. The difference reached significance at the highest dose tested (800 mg/day). Remacemide hydrochloride was well tolerated.
Na(+) channel effects of remacemide and desglycinyl-remacemide in rat cortical synaptosomes.[Pubmed:11906711]
Eur J Pharmacol. 2002 Mar 1;438(1-2):63-8.
The effects of the novel anticonvulsant, Remacemide hydrochloride and its active metabolite, desglycinyl-remacemide, on veratridine-induced Na(+) influx in rat cortical synaptosomes were investigated and compared to established Na(+) channel blocking antiepileptic drugs. Remacemide and desglycinyl-remacemide reduced veratridine-stimulated Na(+) influx to 30.7% (IC(50)=160.6 microM) and 13.2% (IC(50)=85.1 microM) of control, respectively. Carbamazepine, phenytoin and lamotrigine similarly reduced Na(+) influx to 20.1% (IC(50)=325.9 microM), 79.8% and 27.9% (IC(50)=23.0 microM) of control, respectively. Resting internal Na(+) concentrations were significantly increased by desglycinyl-remacemide (1 and 10 microM) and, conversely, decreased by desglycinyl-remacemide and carbamazepine (both 1000 microM). These studies support previous electrophysiological investigations, which suggest that remacemide and desglycinyl-remacemide exert their antiepileptic effects, at least in part, by an inhibitory action on voltage-gated Na(+) channels. Desglycinyl-remacemide may have an additional action on Na(+) homeostasis that merits further exploration.
Block of the N-methyl-D-aspartate receptor by remacemide and its des-glycine metabolite.[Pubmed:8558426]
J Pharmacol Exp Ther. 1996 Jan;276(1):161-8.
The anticonvulsant and neuroprotective properties of remacemide [(+/-)-2-amino-N-(1-methyl-1,2-diphenylethyl)acetamide] and its active des-glycine metabolite [(+/-)-1-methyl-1,2-diphenylethylamine] may result in part from blockade of N-methyl-D-aspartate (NMDA) receptors. The blocking actions of the remacemide enantiomers and their des-glycinates were investigated in whole cell voltage-clamp recordings from cultured rat hippocampal neurons and in binding studies with [3H]dizocilpine in rat forebrain membranes. (+/-)-Remacemide caused a rapid and reversible inhibition of NMDA-evoked current; the R(+)- and S(-)-enantiomers were roughly equipotent (IC50 values at -60 mV, 67 and 75 microM, respectively). In contrast, the block by the S(+)- and R(-)-des-glycine analogs was slower, more potent and occurred in a stereoselective fashion (IC50 values, 0.7 and 4 microM). The block by S(+)-des-glycine remacemide was strongly use- and voltage-dependent, and, in addition, could be occluded by Mg++, indicating that it occurs by an open channel mechanism. In contrast, the block by R(+)-remacemide was only partially voltage-dependent, suggesting that it occurs by both channel blocking and nonchannel blocking (allosteric) mechanisms. Support for an allosteric mechanism was obtained in nonequilibrium [3H]dizocilpine binding studies where it was observed that 100 microM R(+)-remacemide slowed the dissociation of the radioligand [whereas 10 microM S(+)-des-glycine remacemide did not]. Neither R(+)-remacemide nor S(+)-des-glycine remacemide inhibited currents evoked by kainate, alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionate or gamma-aminobutyric acid. We conclude that des-glycine remacemide is a potent and selective channel blocking NMDA receptor antagonist, whereas remacemide is weaker and inhibits NMDA receptors by both channel blocking and nonchannel blocking actions.


