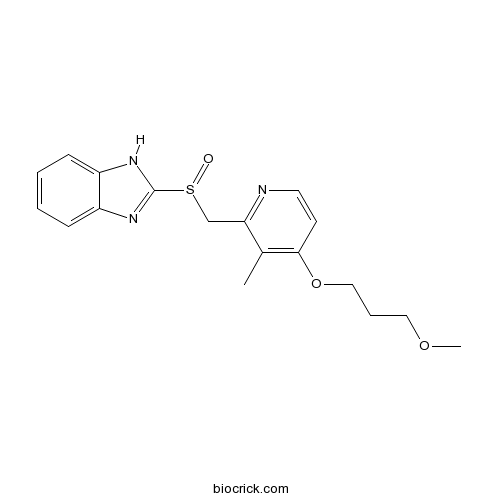
RabeprazoleCAS# 117976-89-3 |
- PRT062607 Hydrochloride
Catalog No.:BCC1869
CAS No.:1370261-97-4
- BAY 61-3606 dihydrochloride
Catalog No.:BCC1407
CAS No.:648903-57-5
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
- R406 (free base)
Catalog No.:BCC2553
CAS No.:841290-80-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117976-89-3 | SDF | Download SDF |
| PubChem ID | 5029 | Appearance | Powder |
| Formula | C18H21N3O3S | M.Wt | 359.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >12.7mg/mL in DMSO | ||
| Chemical Name | 2-[[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylsulfinyl]-1H-benzimidazole | ||
| SMILES | CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3N2)OCCCOC | ||
| Standard InChIKey | YREYEVIYCVEVJK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H21N3O3S/c1-13-16(19-9-8-17(13)24-11-5-10-23-2)12-25(22)18-20-14-6-3-4-7-15(14)21-18/h3-4,6-9H,5,10-12H2,1-2H3,(H,20,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Rabeprazole Dilution Calculator

Rabeprazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7821 mL | 13.9105 mL | 27.8211 mL | 55.6421 mL | 69.5526 mL |
| 5 mM | 0.5564 mL | 2.7821 mL | 5.5642 mL | 11.1284 mL | 13.9105 mL |
| 10 mM | 0.2782 mL | 1.3911 mL | 2.7821 mL | 5.5642 mL | 6.9553 mL |
| 50 mM | 0.0556 mL | 0.2782 mL | 0.5564 mL | 1.1128 mL | 1.3911 mL |
| 100 mM | 0.0278 mL | 0.1391 mL | 0.2782 mL | 0.5564 mL | 0.6955 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Luzindole
Catalog No.:BCC6826
CAS No.:117946-91-5
- GLYX 13
Catalog No.:BCC6013
CAS No.:117928-94-6
- Boc-N-Me-Nle-OH
Catalog No.:BCC2611
CAS No.:117903-25-0
- Forsythoside H
Catalog No.:BCN6431
CAS No.:1178974-85-0
- 7,4'-Dihydroxyhomoisoflavanone
Catalog No.:BCN3582
CAS No.:1178893-64-5
- Fmoc-Thr(Bzl)-OH
Catalog No.:BCC3550
CAS No.:117872-75-0
- L-CCG-lll
Catalog No.:BCC6608
CAS No.:117857-95-1
- L-CCG-l
Catalog No.:BCC6609
CAS No.:117857-93-9
- Loreclezole hydrochloride
Catalog No.:BCC7009
CAS No.:117857-45-1
- Ac-Asp(OtBu)-OH
Catalog No.:BCC2880
CAS No.:117833-18-8
- Enterostatin
Catalog No.:BCC6050
CAS No.:117830-79-2
- 3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No.:BCN8203
CAS No.:1178-24-1
- Rabeprazole sodium
Catalog No.:BCC5227
CAS No.:117976-90-6
- Guanosine
Catalog No.:BCN2962
CAS No.:118-00-3
- Hydrastine
Catalog No.:BCC8187
CAS No.:118-08-1
- Cinchonine
Catalog No.:BCN2464
CAS No.:118-10-5
- Syringin
Catalog No.:BCN6059
CAS No.:118-34-3
- Trimethylgallic acid
Catalog No.:BCN3424
CAS No.:118-41-2
- Maltol
Catalog No.:BCN4819
CAS No.:118-71-8
- Ortho-Hydroxyacetophenone
Catalog No.:BCN3827
CAS No.:118-93-4
- Acetylepipodophyllotoxin
Catalog No.:BCN6056
CAS No.:1180-35-4
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Blumeatin
Catalog No.:BCN6055
CAS No.:118024-26-3
- PHM 27 (human)
Catalog No.:BCC5869
CAS No.:118025-43-7
A multicenter randomized trial comparing rabeprazole and itopride in patients with functional dyspepsia in Japan: the NAGOYA study.[Pubmed:28366993]
J Clin Biochem Nutr. 2017 Mar;60(2):130-135.
The aims of this study were to compare the therapeutic effects of a proton pump inhibitor (PPI), Rabeprazole (RPZ), and a prokinetic agent, itopride (ITO), and to investigate the role of PPI in the treatment strategy for Japanese functional dyspepsia (FD) patients. We randomly assigned 134 patients diagnosed by Rome III criteria to 4 weeks treatment with RPZ 10 mg/day (n = 69) or ITO 150 mg/day (n = 65). Dyspeptic symptoms were evaluated using FD scores at baseline and after 1, 2 and 4 weeks of treatment. We also divided subjects into predominantly epigastric pain syndrome (EPS) or postprandial distress syndrome (PDS), and evaluated the efficacy of RPZ and ITO respectively. RPZ showed a significant decrease in the Rate of Change (RC) in FD score within 1 week, which was maintained until after 4 weeks, with RPZ a significant effect compared with ITO at all evaluation points. In addition, RPZ showed a significant decrease in FD score in subjects with both EPS and PDS, whereas a significant decrease in the RC with ITO was only shown in those with predominant PDS. Acid-suppressive therapy with RPZ is useful for PDS as well EPS in Japanese FD patients (UMIN Clinical Trials Registry number: UMIN 000013962).
Stereoselective and nonstereoselective pharmacokinetics of rabeprazole - an overview.[Pubmed:28294690]
Xenobiotica. 2018 Apr;48(4):422-432.
1. Proton pump inhibitors have been extensively used for the treatment of ailments due to increased gastric acid secretion such as peptic ulcers, gastroesophageal reflux disease, etc. 2. There are several approved drugs in the proton pump inhibitor class with the latest entries representing single enantiomer drugs of the previously approved racemic drugs. 3. Despite having a high degree of structural resemblance, Rabeprazole, was shown to possess some unique differentiation from other drugs in the class. One of the key distinguishing features of Rabeprazole was related to the lesser involvement of polymorphic metabolism in its pharmacokinetic disposition. 4. The review was aimed to provide pharmacokinetic data of Rabeprazole from several clinical studies including drug-drug interaction studies where Rabeprazole was either a perpetrator drug or victim drug. 5. Additional perspectives on therapy considerations due to the unique metabolic disposition of Rabeprazole including the possible issues related to chirality were provided.
Determination of rabeprazole enantiomers in commercial tablets using immobilized cellulose-based stationary phase.[Pubmed:28229392]
Arch Pharm Res. 2017 Mar;40(3):373-381.
Rabeprazole is one of the latest proton-pump inhibitors used for treatment of several gastrointestinal disorders. For therapeutic applications, Rabeprazole has been administered as a mixture of R-(+) and S-(-) enantiomers. Owing to pharmacological and toxicological differences between stereoisomers, chiral recognition has now become an integral part of drug research and development. A simple and rapid liquid chromatographic method for enantioselective separation and determination of R-(+) and S-(-) enantiomers of Rabeprazole in bulk drug and pharmaceutical formulations was developed. Chiralpak IC (150 x 4.6 mm, 5 mum) column and mumobile phase containing hexane:ethanol:ethylenediamine (30:70:0.05 v/v) in an isocratic mode yielded baseline separation with resolution greater than 6.0 at 35 degrees C. Effects of additives and n-hexane were evaluated. Optimized condition was validated as per ICH guidelines. The method has good linearity, high sensitivity with LOD was 0.01 mug/mL and LOQ was 0.03 mug/mL for both enantiomers. Intra-day precision varied between 0.44 and 1.79% for S-(-) enantiomer, 0.65 and 1.97% for R-(+) enantiomer. Relative standard deviations of inter-day precision were less than 1.81% for both enantiomers. The percentage recovery for both enantiomers of Rabeprazole ranged between 99.81 and 101.95%, 98.82 and 101.36% in material and tablets, respectively. The method was successfully applied to determine content of each enantiomer in commercial tablets.
A Multicenter, Randomized, Open-Label Trial: Efficacy of Once-Daily Versus Twice-Daily Double-Dose Rabeprazole on Refractory Gastroesophageal Reflux Disease-Related Symptoms and Quality of Life.[Pubmed:28066515]
Curr Ther Res Clin Exp. 2016 Nov 25;79:1-7.
BACKGROUND: Approximately 20% to 40% of patients with gastroesophageal reflux disease (GERD) are refractory to standard-dose proton-pump inhibitor (PPI) treatment. OBJECTIVE: We compared the efficacy and quality-of-life effects of 20 mg once daily (QD) versus 10 mg twice daily (BID) Rabeprazole (RPZ) in patients with refractory GERD-related symptoms and sleep disturbances. METHODS: This multicenter, prospective, randomized, open-label study included patients in whom PPI treatment >4 weeks was ineffective. According to the Global Overall Symptom (GOS) scale, PPI-refractory GERD was defined as >/=1 category with >3 points among 10 specific upper gastrointestinal symptoms. Seventy-eight patients were randomly assigned to 20 mg QD and 10 mg BID RPZ groups for 8 weeks. Efficacy was evaluated using self-reported questionnaires, including the GOS scale and Pittsburg Sleep Quality Index (PSQI), whereas quality of life was assessed using the Short-Form 8 Health Survey (SF-8), at 4 and 8 weeks. Patients showing improvement at 8 weeks received follow-up every 4 to 8 weeks. RESULTS: GOS scale scores were significantly improved at 8 weeks in both groups, with no significant intergroup differences. Although SF-8 scores showed an increasing trend over 8 weeks in both groups, the physical component summaries in the 10 mg BID group significantly improved. The mental component summaries clearly improved in the 10 mg BID group. Of the 74 cases (4 missing), 51 (68.9%) had PSQI scores >/=5.5. PSQI scores remained unchanged during follow-up in both groups. The recurrence rate was not significantly different (46.1% vs 47.1% in the 20 mg QD and 10 mg BID groups, respectively) during the follow-up period at median (interquartile range) 24.0 (30.5) months. CONCLUSIONS: In patients with refractory GERD, there was no significant difference in GOS scale score, PSQI, or recurrence rate between the groups. With regard to subscores of the SF-8, the 10 mg BID group might be potentially effective.


