RWJ 52353CAS# 245744-10-9 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 245744-10-9 | SDF | Download SDF |
| PubChem ID | 9815610 | Appearance | Powder |
| Formula | C11H10N2S | M.Wt | 202.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 50 mM in ethanol | ||
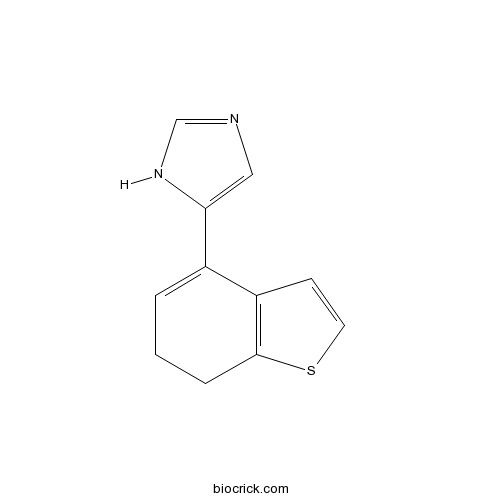
| Chemical Name | 5-(6,7-dihydro-1-benzothiophen-4-yl)-1H-imidazole | ||
| SMILES | C1CC2=C(C=CS2)C(=C1)C3=CN=CN3 | ||
| Standard InChIKey | BAADWHDQAKDYLX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H10N2S/c1-2-8(10-6-12-7-13-10)9-4-5-14-11(9)3-1/h2,4-7H,1,3H2,(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Agonist of the α2D adrenergic receptor (Ki values are 1.5, 254, 443 and 621 nM for α2D, α2A, α1 and α2B adrenergic receptors respectively). |

RWJ 52353 Dilution Calculator

RWJ 52353 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.9436 mL | 24.7182 mL | 49.4364 mL | 98.8728 mL | 123.5911 mL |
| 5 mM | 0.9887 mL | 4.9436 mL | 9.8873 mL | 19.7746 mL | 24.7182 mL |
| 10 mM | 0.4944 mL | 2.4718 mL | 4.9436 mL | 9.8873 mL | 12.3591 mL |
| 50 mM | 0.0989 mL | 0.4944 mL | 0.9887 mL | 1.9775 mL | 2.4718 mL |
| 100 mM | 0.0494 mL | 0.2472 mL | 0.4944 mL | 0.9887 mL | 1.2359 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Muscarine iodide
Catalog No.:BCC7556
CAS No.:24570-49-8
- 5'-S-Methyl-5'-thioadenosine
Catalog No.:BCN5108
CAS No.:2457-80-9
- 7,8,9-Trimethoxy-10H-1,3-dioxolo[4,5-b]xanthen-10-one
Catalog No.:BCN1477
CAS No.:24562-58-1
- (2S,3R,E)-2-Amino-4-tetradecene-1,3-diol
Catalog No.:BCN1478
CAS No.:24558-60-9
- Decursitin D
Catalog No.:BCN3908
CAS No.:245446-61-1
- LED209
Catalog No.:BCC6437
CAS No.:245342-14-7
- FSLLRY-NH2
Catalog No.:BCC6279
CAS No.:245329-02-6
- LRGILS-NH2
Catalog No.:BCC3955
CAS No.:245329-01-5
- Makisterone A 20,22-monoacetonide
Catalog No.:BCN7090
CAS No.:245323-24-4
- 1,2-Dioleoyl-sn-glycerol
Catalog No.:BCC6416
CAS No.:24529-88-2
- 2-Cyano-N-[4-(Trifluoromethyl)Phenyl]Acetamide
Catalog No.:BCC8571
CAS No.:24522-30-3
- n-Tritriacontan-16,18-dione
Catalog No.:BCC9106
CAS No.:24514-86-1
- TC-G 1000
Catalog No.:BCC7991
CAS No.:245744-18-7
- Rubrofusarin-6-O-beta-D-gentiobioside
Catalog No.:BCN1476
CAS No.:24577-90-0
- trans-4-Hydroxycrotonic acid
Catalog No.:BCC6685
CAS No.:24587-49-3
- BIIE 0246
Catalog No.:BCC7158
CAS No.:246146-55-4
- H-Phe-OBzl.HCl
Catalog No.:BCC3006
CAS No.:2462-32-0
- AL 8810
Catalog No.:BCC5366
CAS No.:246246-19-5
- 3-Geranyl-4-methoxybenzoic acid
Catalog No.:BCN5109
CAS No.:246266-38-6
- H-Isoleucinol
Catalog No.:BCC2726
CAS No.:24629-25-2
- Stellasterol
Catalog No.:BCN5110
CAS No.:2465-11-4
- Neolitsine
Catalog No.:BCN4817
CAS No.:2466-42-4
- 3-Amino-2,6-piperidinedione hydrochloride
Catalog No.:BCC8606
CAS No.:24666-56-6
- Catharanthine
Catalog No.:BCN1255
CAS No.:2468-21-5
Identification of an active metabolite of PAR-1 antagonist RWJ-58259 and synthesis of analogues to enhance its metabolic stability.[Pubmed:26927018]
Org Biomol Chem. 2016 Mar 28;14(12):3198-201.
The discontinuation of PAR-1 antagonist RWJ-58259 beyond use as a biological probe is most likely due to it's short half-life in vivo. However, retention of significant in vivo activity beyond the point where most of the RWJ-58259 had been consumed implies the generation of an active metabolite. Herein we describe the biological activity of a predicted metabolite of RWJ-58259 and the synthesis of analogues designed to enhance the metabolic stability of RWJ-58259.
Leveraging Academic-Service Partnerships: Implications for Implementing the RWJ/IOM's Recommendations to Improve Quality, Access, and Value in Academic Medical Centers.[Pubmed:22191053]
ISRN Nurs. 2011;2011:731902.
Transformation of the current healthcare system is critical to achieve improved quality, safety, value, and access. Patients with multiple, chronic health conditions require integrated care coordination yet the current health care system is fragmented and complex. Nursing must play a key role in constructing a system that is value based and patient focused. The Robert Wood Johnson/Institute of Medicine (RWJ/IOM) report on the future of nursing outlines strategic opportunities for nursing to take a lead role in this transformation. Partnerships across academic institutions and health care systems have the potential to address issues through mutual goal setting, sharing of risks, responsibilities, and accountability, and realignment of resources. The purpose of this paper is to present Stony Brook University Medical Center's (SBUMC) academic-service partnership which implemented several of the RWJ/IOM recommendations. The partnership resulted in several initiatives that improved quality, safety, access, and value. It also characterized mutual goal setting, shared missions and values, and a united vision for health care.
Synergistic Effects of Transplanted Endothelial Progenitor Cells and RWJ 67657 in Diabetic Ischemic Stroke Models.[Pubmed:26045601]
Stroke. 2015 Jul;46(7):1938-46.
BACKGROUND AND PURPOSE: An immature vascular phenotype in diabetes mellitus may cause more severe vascular damage and poorer functional outcomes after stroke, and it would be feasible to repair damaged functional vessels using endothelial progenitor cell (EPC) transplantation. However, high glucose induces p38 mitogen-activated protein kinase activation, which can accelerate the senescence and apoptosis of EPCs. The aim of this study was to investigate the combined effects of EPC transplantation and p38 mitogen-activated protein kinase inhibitor administration on diabetic stroke outcomes. METHODS: Bone marrow-derived EPCs were injected intra-arterially into db/db mice after ischemic stroke induction. RWJ 67657 (RWJ), a p38 mitogen-activated protein kinase inhibitor, was administered orally for 7 consecutive days, with the first dose given 30 minutes before stroke induction. Functional outcome was determined at days 0, 1, 7, 14, and 21. Angiogenesis, neurogenesis, infarct volume, and Western blotting assays were performed on day 7, and white matter remodeling was determined on day 14. RESULTS: Neither EPC transplantation nor RWJ administration alone significantly improved diabetic stroke outcome although RWJ displayed a potent anti-inflammatory effect. By both improving the functioning of EPCs and reducing inflammation, EPC transplantation plus RWJ administration in vivo synergistically promoted angiogenesis and neurogenesis after diabetic stroke. In addition, the white matter remodeling, behavioral scores, and expressions of vascular endothelial growth factor and brain-derived neurotrophic factor were significantly increased in diabetic mice treated with both EPCs and RWJ. CONCLUSIONS: The combination of EPC transplantation and RWJ administration accelerated recovery from diabetic stroke, which might have been caused by increased levels of proangiogenic and neurotrophic factors.
Inhibitory and facilitory actions of isocyanine derivatives at human and rat organic cation transporters 1, 2 and 3: a comparison to human alpha 1- and alpha 2-adrenoceptor subtypes.[Pubmed:20170649]
Eur J Pharmacol. 2010 May 25;634(1-3):1-9.
Organic cation transporters (OCTs), comprising OCT1, OCT2 and OCT3 subtypes, control absorption and elimination of xenobiotics and endogenous compounds in kidney, liver and placenta. In addition, they ensure "uptake2", low-affinity catecholamine clearance in sympathetically-innervated tissue and the CNS. The prototypical OCT ligand, disprocynium24 (D24), recognises OCT3, but its actions at OCT1 and OCT2 remain unknown. Herein, together with two other isocyanine derivatives (AAC291 and AAC301) and chemically-related adrenergic agents, we evaluated actions of D24 at OCTs, monoamine transporters and alpha(1)- and alpha(2)-adrenoceptors. D24 concentration-dependently suppressed [3H]-1-methyl-4-phenylpyridinium (MPP+) transport at human (h) and rat (r) OCT1, OCT2 and OCT3 in stably transfected HEK293 cells. Interestingly, low concentrations of D24 enhanced transport by h/rOCT2, a substrate-dependent effect suppressed by inhibition of protein kinase C. AAC291 and AAC301 likewise inhibited transport by all classes of h/r OCT and at low concentrations induced even more marked increases in transport by h/rOCT2. Further, by analogy to D24, they displayed antagonist properties at halpha(1A/B/D)-adrenoceptors (Ca2+-flux) and halpha(2A/B/C)-adrenoceptors ([35S]GTPgammaS binding). They were, however, less potent than D24 at serotonin transporters ([3H]citalopram binding) and AAC291 did not bind to dopamine and norepinephrine transporters. The preferential alpha(1B)-adrenoceptor antagonist, AH11110A, the alpha2-adrenoceptor agonist, RWJ52353, and the adrenergic neurotoxin DSP-4 likewise affected [3H]MPP+ transport, in an OCT-subtype and species-dependent manner. In conclusion, D24, other isocyanine congeners and chemically-related adrenergic agents inhibit OCT-mediated [3H]MPP+ transport, and all drugs display significant activity at alpha1- and alpha2-adrenoceptor subtypes, expanding previous reports of promiscuity between pharmacophores recognising alpha-adrenoceptors and OCTs.


