QuestinCAS# 3774-64-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3774-64-9 | SDF | Download SDF |
| PubChem ID | 160717 | Appearance | Powder |
| Formula | C16H12O5 | M.Wt | 284.26 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
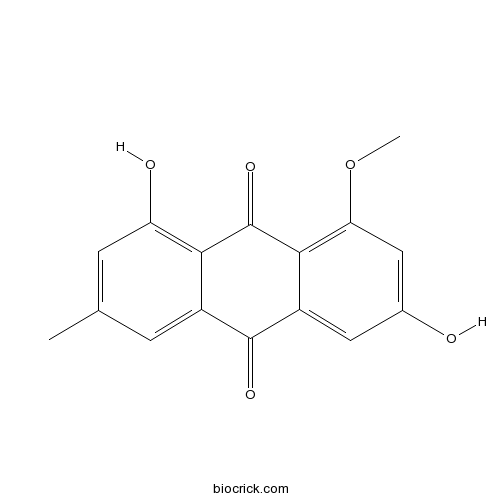
| Chemical Name | 1,6-dihydroxy-8-methoxy-3-methylanthracene-9,10-dione | ||
| SMILES | CC1=CC(=C2C(=C1)C(=O)C3=CC(=CC(=C3C2=O)OC)O)O | ||
| Standard InChIKey | UUNPIWCQMVNINR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O5/c1-7-3-9-13(11(18)4-7)16(20)14-10(15(9)19)5-8(17)6-12(14)21-2/h3-6,17-18H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Questin has promising inhibitory potential against AChE, BChE, and BACE1, it may be used in the development of therapeutic or preventive agents for Alzheimer's disease. 2. Questin is toxic to the human A549 lung cell line. 3. Questin is a Cdc25B phosphatase inhibitor, it inhibited the enzymatic activity of Cdc25B phosphatase with the IC(50) value of 34 microg/mL, it also strongly inhibited the growth of human colon cancer cells, SW620 with the GI(50) value of 0.9 microg/mL. 4. Questin shows considerably high immunosuppressive activity. |
| Targets | AChR | BChE | NO | Immunology & Inflammation related |

Questin Dilution Calculator

Questin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5179 mL | 17.5895 mL | 35.1791 mL | 70.3581 mL | 87.9477 mL |
| 5 mM | 0.7036 mL | 3.5179 mL | 7.0358 mL | 14.0716 mL | 17.5895 mL |
| 10 mM | 0.3518 mL | 1.759 mL | 3.5179 mL | 7.0358 mL | 8.7948 mL |
| 50 mM | 0.0704 mL | 0.3518 mL | 0.7036 mL | 1.4072 mL | 1.759 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3518 mL | 0.7036 mL | 0.8795 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Chloro-N6-cyclopentyladenosine
Catalog No.:BCC7161
CAS No.:37739-05-2
- Boc-Cha-OH
Catalog No.:BCC2661
CAS No.:37736-82-6
- Rhodojaponin V
Catalog No.:BCN2807
CAS No.:37720-86-8
- Totaradiol
Catalog No.:BCN5431
CAS No.:3772-56-3
- Dehydroabietinol
Catalog No.:BCN5430
CAS No.:3772-55-2
- SU 9516
Catalog No.:BCC2398
CAS No.:377090-84-1
- 8-Acetonyldihydrosanguinarine
Catalog No.:BCN5429
CAS No.:37687-34-6
- LY450108
Catalog No.:BCC1725
CAS No.:376594-67-1
- BRD 7389
Catalog No.:BCC8090
CAS No.:376382-11-5
- 1-(4-Hydroxy-2-methoxyphenyl)-3-(4-hydroxy-3-prenylphenyl)propane
Catalog No.:BCN1450
CAS No.:376362-03-7
- 4'-O-Demethylbroussonin A
Catalog No.:BCN7364
CAS No.:376361-97-6
- 7,4'-Dihydroxy-3'-prenylflavan
Catalog No.:BCN5428
CAS No.:376361-96-5
- Iristectorin A
Catalog No.:BCN8221
CAS No.:37744-61-9
- Enmein
Catalog No.:BCN3392
CAS No.:3776-39-4
- Zaprinast
Catalog No.:BCC6859
CAS No.:37762-06-4
- 2-Pentylfuran
Catalog No.:BCN3799
CAS No.:3777-69-3
- Preladenant
Catalog No.:BCC1868
CAS No.:377727-87-2
- 7''-O-Methylsciadopitysin
Catalog No.:BCN4018
CAS No.:3778-25-4
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
- Boc-D-Pro-OH
Catalog No.:BCC3437
CAS No.:37784-17-1
- Cimigenol
Catalog No.:BCC8149
CAS No.:3779-59-7
- Betamethasone
Catalog No.:BCC4765
CAS No.:378-44-9
- ACDPP hydrochloride
Catalog No.:BCC7302
CAS No.:37804-11-8
- Phaseollidin
Catalog No.:BCN5432
CAS No.:37831-70-2
Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against beta-secretase and cholinesterases.[Pubmed:27321278]
J Ethnopharmacol. 2016 Sep 15;191:152-160.
ETHNOPHARMACOLOGICAL RELEVANCE: Semen Cassiae has been traditionally used as an herbal remedy for liver, eye, and acute inflammatory diseases. Recent pharmacological reports have indicated that Cassiae semen has neuroprotective effects, attributable to its anti-inflammatory actions, in ischemic stroke and Alzheimer's disease (AD) models. AIM OF THE STUDY: The basic goal of this study was to evaluate the anti-AD activities of C. obtusifolia and its major constituents. Previously, the extract of C. obtusifolia seeds, was reported to have memory enhancing properties and anti-AD activity to ameliorate amyloid beta-induced synaptic dysfunction. However, the responsible components of C. obtusifolia seeds in an AD are currently still unknown. In this study, we investigated the inhibitory effects of C. obtusifolia and its constituents against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and beta-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) enzyme activity. MATERIALS AND METHODS: In vitro cholinesterase enzyme assays by using AChE, BChE, and BACE1 were performed. We also scrutinized the potentials of Cassiae semen active component as BACE1 inhibitors via enzyme kinetics and molecular docking simulation. RESULTS: In vitro enzyme assays demonstrated that C. obtusifolia and its major constituents have promising inhibitory potential against AChE, BChE, and BACE1. All Cassiae semen constituents exhibited potent inhibitory activities against AChE and BACE1 with IC50 values of 6.29-109microg/mL and 0.94-190microg/mL, whereas alaternin, Questin, and toralactone gentiobioside exhibited significant inhibitory activities against BChE with IC50 values of 113.10-137.74microg/mL. Kinetic study revealed that alaternin noncompetitively inhibited, whereas cassiaside and emodin showed mixed-type inhibition against BACE1. Furthermore, molecular docking simulation results demonstrated that hydroxyl group of alaternin and emodin tightly interacted with the active site residues of BACE1 and their relevant binding energies (-6.62 and -6.89kcal/mol), indicating a higher affinity and tighter binding capacity of these compounds for the active site of BACE1. CONCLUSION: The findings of the present study suggest the potential of C. obtusifolia and its major constituents for use in the development of therapeutic or preventive agents for AD, especially through inhibition of AChE, BChE and BACE1 activities.
Immunomodulatory constituents from an ascomycete, Microascus tardifaciens.[Pubmed:10553639]
Chem Pharm Bull (Tokyo). 1999 Oct;47(10):1426-32.
Fractionation guided by the immunosuppressive activity of the defatted AcOEt extract of an Ascomycete, Microascus tardifaciens, afforded eight constituents, Questin (emodin 8-O-methylether) (1), rubrocristin (2), 5,7-dihydroxy-4-methylphthalide (3), cladosporin (asperentin) (4), cladosporin 8-O-methylether (5), tradioxopiperazine A [cyclo-L-alanyl-5-isopentenyl-2-(1',1'-dimethylallyl)-L-tryptophan] (6), tradioxopiperazine B [cyclo-L-alanyl-7-isopentenyl-2-(1',1'-dimethylallyl)-L-tryptophan] (7), and asperflavin (8), among which 6 and 7 were new compounds. Compounds 1 and 2 showed considerably high immunosuppressive activity, 6 was moderate and, 3, 4, 5, 7 and 8 showed low activity.
Anthraquinones, Cdc25B phosphatase inhibitors, isolated from the roots of Polygonum multiflorum Thunb.[Pubmed:17497420]
Nat Prod Res. 2007 May 20;21(6):487-93.
Three anthraquinones, Cdc25B phosphatase inhibitors, were isolated from the methanolic extract of the roots of Polygonum multiflorum Thunb. (Polygonaceae). Anthraquinones, physcion (1), emodin (2), and Questin (3), inhibited the enzymatic activity of Cdc25B phosphatase with IC(50) values of 62.5, 30, and 34 microg mL(-1), respectively. Emodin (2) and Questin (3) strongly inhibited the growth of human colon cancer cells, SW620 with GI(50) values of 6.1 and 0.9 microg mL(-1), respectively. Commercially available anthraquinones, chrysophanol (4), and rhein (5) also inhibited Cdc25B phosphatase with IC(50) values of 10.7 and 22.1 microg mL(-1), respectively.
Trypacidin, a spore-borne toxin from Aspergillus fumigatus, is cytotoxic to lung cells.[Pubmed:22319557]
PLoS One. 2012;7(2):e29906.
Inhalation of Aspergillus fumigatus conidia can cause severe aspergillosis in immunosuppressed people. A. fumigatus produces a large number of secondary metabolites, some of which are airborne by conidia and whose toxicity to the respiratory tract has not been investigated. We found that spores of A. fumigatus contain five main compounds, tryptoquivaline F, fumiquinazoline C, Questin, monomethylsulochrin and trypacidin. Fractionation of culture extracts using RP-HPLC and LC-MS showed that samples containing Questin, monomethylsulochrin and trypacidin were toxic to the human A549 lung cell line. These compounds were purified and their structure verified using NMR in order to compare their toxicity against A549 cells. Trypacidin was the most toxic, decreasing cell viability and triggering cell lysis, both effects occurring at an IC(5)(0) close to 7 microM. Trypacidin toxicity was also observed in the same concentration range on human bronchial epithelial cells. In the first hour of exposure, trypacidin initiates the intracellular formation of nitric oxide (NO) and hydrogen peroxide (H(2)O(2)). This oxidative stress triggers necrotic cell death in the following 24 h. The apoptosis pathway, moreover, was not involved in the cell death process as trypacidin did not induce apoptotic bodies or a decrease in mitochondrial membrane potential. This is the first time that the toxicity of trypacidin to lung cells has been reported.


