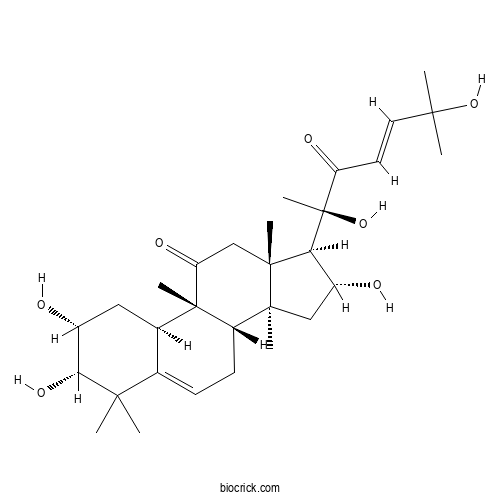
PresenegeninCAS# 2163-40-8 |
- Cucurbitacin F
Catalog No.:BCN0918
CAS No.:5939-57-1
- Cucurbitacin O
Catalog No.:BCN0921
CAS No.:25383-23-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2163-40-8 | SDF | Download SDF |
| PubChem ID | 5281322.0 | Appearance | Powder |
| Formula | C30H46O7 | M.Wt | 518.69 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S,8S,9R,10R,13R,14S,16R,17R)-17-[(E,2R)-2,6-dihydroxy-6-methyl-3-oxohept-4-en-2-yl]-2,3,16-trihydroxy-4,4,9,13,14-pentamethyl-1,2,3,7,8,10,12,15,16,17-decahydrocyclopenta[a]phenanthren-11-one | ||
| SMILES | CC1(C(C(CC2C1=CCC3C2(C(=O)CC4(C3(CC(C4C(C)(C(=O)C=CC(C)(C)O)O)O)C)C)C)O)O)C | ||
| Standard InChIKey | AOHIGMQGPFTKQX-SSGGGWRTSA-N | ||
| Standard InChI | InChI=1S/C30H46O7/c1-25(2,36)12-11-21(33)30(8,37)23-19(32)14-27(5)20-10-9-16-17(13-18(31)24(35)26(16,3)4)29(20,7)22(34)15-28(23,27)6/h9,11-12,17-20,23-24,31-32,35-37H,10,13-15H2,1-8H3/b12-11+/t17-,18-,19-,20+,23+,24-,27+,28-,29+,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Presenegenin Dilution Calculator

Presenegenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9279 mL | 9.6397 mL | 19.2793 mL | 38.5587 mL | 48.1983 mL |
| 5 mM | 0.3856 mL | 1.9279 mL | 3.8559 mL | 7.7117 mL | 9.6397 mL |
| 10 mM | 0.1928 mL | 0.964 mL | 1.9279 mL | 3.8559 mL | 4.8198 mL |
| 50 mM | 0.0386 mL | 0.1928 mL | 0.3856 mL | 0.7712 mL | 0.964 mL |
| 100 mM | 0.0193 mL | 0.0964 mL | 0.1928 mL | 0.3856 mL | 0.482 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ligustrosidic acid
Catalog No.:BCX1140
CAS No.:96382-89-7
- 3α-Hydroxymogrol
Catalog No.:BCX1139
CAS No.:1343402-73-2
- N-methyltyramine
Catalog No.:BCX1138
CAS No.:370-98-9
- Ganosporeric acid A
Catalog No.:BCX1137
CAS No.:135357-25-4
- Avenanthramide A
Catalog No.:BCX1136
CAS No.:108605-70-5
- Avenanthramide B
Catalog No.:BCX1135
CAS No.:108605-69-2
- 3-Feruloyl-4-caffeoylquinic acid
Catalog No.:BCX1134
CAS No.:96990-65-7
- β-Sitosteryl acetate
Catalog No.:BCX1133
CAS No.:915-05-9
- Tetraacetylphytosphingosine
Catalog No.:BCX1132
CAS No.:13018-48-9
- L-Guluronic Acid Sodium Salt
Catalog No.:BCX1131
CAS No.:15769-56-9
- Ostruthine
Catalog No.:BCX1130
CAS No.:148-83-4
- Agigenin
Catalog No.:BCX1129
CAS No.:55332-76-8
- Hydroxypropyl tetrahydropyrantriol
Catalog No.:BCX1142
CAS No.:439685-79-7
- Euphornin
Catalog No.:BCX1143
CAS No.:80454-47-3
- Reptoside
Catalog No.:BCX1144
CAS No.:53839-03-5
- Jaligonic acid
Catalog No.:BCX1145
CAS No.:51776-39-7
- 24(28)-Dehydroergosterol
Catalog No.:BCX1146
CAS No.:29560-24-5
- Eicosapentaenoic acid
Catalog No.:BCX1147
CAS No.:10417-94-4
- Toralactone
Catalog No.:BCX1148
CAS No.:41743-74-2
- Rubropunctatin
Catalog No.:BCX1149
CAS No.:514-67-0
- Methyl brevifolincarboxylate
Catalog No.:BCX1150
CAS No.:154702-76-8
- Butyl neochlorogenate
Catalog No.:BCX1151
CAS No.:409361-64-4
- Butyl chlorogenate
Catalog No.:BCX1152
CAS No.:132741-56-1
- Spicatine A
Catalog No.:BCX1153
CAS No.:124256-81-1
Preparation of Tenuifolin from Polygala senega L. Root Using a Hydrolytic Continuous Flow System under High-Temperature, High-Pressure Conditions.[Pubmed:34730980]
J Org Chem. 2021 Dec 3;86(23):16268-16277.
An improved process for preparing tenuifolin (Presenegenin 3-beta-d-glucopyranoside) from the root of Polygala senega L. was developed. A crude saponin mixture extracted from P. senega was subjected to hydrolysis, and the reactivity of compounds in the extract was controlled by utilizing the combination of a flow reactor and experimental design. In addition, column chromatography with HP 20, a synthetic polystyrenic adsorbent, allowed the gram-scale preparation of tenuifolin in a continuous manner with fewer steps. This approach shortens the total time required for gram-scale preparation from 16 to 5 h in a continuous manner while improving the yield from 0.59% to 2.08% (w/w).
[Saponins from roots of Securidaca inappendiculata with cytotoxic activities].[Pubmed:26666038]
Zhongguo Zhong Yao Za Zhi. 2015 Jul;40(14):2849-53.
Seven acylated triterpene saponins were isolated from the roots of Securidaca inappendiculata by means of various chromatographic techniques such as silica gel, MPLC, preparative HPLC, and semi-preparative HPLC. Their chemical structures were identified as securioside A(1), securioside B(2), 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyranosyl-(1-->3)]-4-O-[(E)-3,4-dimethoxycinnamoyl]-beta-D-fucopyranosyl ester(3), 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyranosyl-(1-->3) ] -4-O-[(E/Z)-3, 4-dimethoxycinnamoyl]-beta-D-fucopyranosyl ester(3/4), 3-O-beta-D-glucopyranosyl Presenegenin 28-O-alpha-L-arabinopyranosyl-(1-->3)-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-4-O-[(E)-3,4-dimethoxycinnamoyl]-beta-D-fucopyranosyl ester(5), polygalasa- ponin XLV(6), and polygalasaponin XLVI (7) on the basis of spectroscopic data analysis and physicochemical properties. Among them, compounds 5-7 were isolated from the plants in genus Securidaca for the first time and compounds 3, 3/4 were isolated from the species for the first time. The cytotoxicity assay showed that compounds 2, 3/4, 5 have moderate cytotoxic activities against Lewis lung carcinoma LLC cells with IC50 values of 41.10, 38.17, and 48.92 micromol . L(-1), respectively; compound 2 exhibited moderate cytotoxic activities against human breast cancer MCF-7 cells with an IC50 value of 47.93 micromol . L(-1).
Chemical investigation of the roots of Polygala sibirica L.[Pubmed:24702811]
Chin J Nat Med. 2014 Mar;12(3):225-8.
AIM: To investigate the chemical constituents of the roots of Polygala sibirica L. (Polygalaceae) METHOD: The isolation was performed by solvent extraction and various chromatographic techniques, including silica gel, Sephadex LH-20, ODS, semi-preparative HPLC, and preparative TLC. The chemical structures were elucidated based on extensive spectroscopic analysis, including HR-ESI-MS and 1D- and 2D-NMR spectroscopic data. RESULTS: A total of sixteen compounds, including five xanthones (5, 7-10), five saccharide esters (1, 3, 4, 12, 13), two flavonoids (14, 16), two triterpenoids (11, 15), one phenylpropanoid (6), and one benzophenone glycoside (2) were isolated. Their structures were determined as sibiricose A7 (1), sibiriphenone A (2), polygalatenoside A (3), polygalatenoside C (4), lancerin (5), 3, 4, 5-trimethoxycinnamic acid (6), 6-hydroxy-1, 2, 3, 7-tetramethoxyxanthone (7), 1, 3, 7-trihydroxy-2-methoxyxanthone (8), onjixanthone II (9), 1, 2, 3, 6, 7-pentamethoxyxanthone (10), Presenegenin (11), 3'-O-3, 4, 5-trimethoxycinnamoyl-6-O-4-methoxy benzoyl sucrose (12), tenuifoliside C (13), 5, 3'-dihydroxy-7, 4'-dimethoxyflavonol-3-O-beta-D-glucopyranoside (14), tenuifolin (15), and rhamnetin 3-O-beta-D-glucopyranoside (16). CONCLUSION: Compounds 1 and 2 are two new compounds from P. sibirica.
[Germplasm resources of Polygala tenuifolia and determination of presenegenin in processing products].[Pubmed:19382450]
Zhongguo Zhong Yao Za Zhi. 2009 Jan;34(1):50-3.
OBJECTIVE: To research the germplasm resources and the contents of senegenin in processing products of Polygala tenuifolia. METHOD: The contents of senegenin in wild Polygala tenuifolia and cultivated samples of Polygala tenuifolia were determined by RP-HPLC, and compared. RESULT: The contents of senegenin in wild reduce gradually along Shaanxi, Shanxi, Hebei to Dongbei. The contents of senegenin in cultivated three-year samples of three year Polygala tenuifolia from five main place was similar, 0.44%-0.49%. The content of senegenin were 0.44%-0.64% in the wand and 0.03%-0.09% in the residual part of stem, and the content of senegenin in Polygala tenuifolia was more than that in processing products. CONCLUSION: There is a correlation between the content of senegenin in Polygala tenuifolia and ecology environment that show a is inverse proportion with the quality grade, and the contents in the processing products were decreased. Senegenin can be used as a characteristic marker in range. This research provides a reference for search a index for quality control of Radix polygala and its processing products.
A novel triterpenoid saponin from Polygala tenuifolia Willd.[Pubmed:18696336]
J Asian Nat Prod Res. 2008 Jul-Aug;10(7-8):813-6.
A new triterpenoid saponin, tenuifoside A, was isolated together with three known triterpenoid saponins 2, 3, and 4 from the roots of Polygala tenuifolia Willd. With the help of chemical and spectral analyses (IR, MS, 1D-NMR, and 2D-NMR), the structure of the new saponin was elucidated as 3-O-beta-d-glucopyranosyl Presenegenin 28-O-beta-d-xylopyranosyl-(1 --> 3)-beta-d-xylopyranosyl-(1 --> 4)-[beta-D-apiofuranosyl-(1 --> 3)]-alpha-L-rhamnopyranosyl-(1 --> 2)-[4-O-p-methoxycinnamoyl]-[alpha-l-rhamnopyranosyl-(1 --> 3)]-beta-d-fucopyranosyl ester (1). Three known triterpenoid saponins (2-4) were identified on the basis of spectroscopic data.
Oligosaccharide polyester and triterpenoid saponins from the roots of Polygala japonica.[Pubmed:18353407]
Phytochemistry. 2008 May;69(7):1617-24.
An oligosaccharide polyester, 1-O-(E)-p-coumaroyl-(3-O-benzoyl)-beta-D-fructofuranosyl-(2-->1)-[6-O-(E)-feruloyl-beta-D-glucopyranosyl-(1-->2)]-[6-O-acetyl-beta-D-glucopyranosyl-(1-->3)-(4-O-acetyl)-beta-D-glucopyranosyl-(1-->3)]-4-O-[4-O-alpha-L-rhamnopyranosyl-(E)-p-coumaroyl]-alpha-D-glucopyranoside (polygalajaponicose I), and four triterpenoid saponins, 3beta, 23, 27-trihydroxy-29-O-beta-D-glucopyranosyl-(1-->2)-beta-D-glucopyranosyl-olean-12-en-28-oic acid (polygalasaponin XLVII), 3-O-beta-D-glucopyranosyl Presenegenin 28-O-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-fucopyranosyl ester (polygalasaponin XLVIII), 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->5)-beta-D-apiofuranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranosyl ester (polygalasaponin XLIX) and 2beta, 27-dihydroxy-3-O-beta-D-glucopyranosyl 11-oxo-olean-12-en-23, 28-dioic acid 28-O-beta-D-galactopyranosyl-(1-->5)-beta-D-apiofuranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-beta-D-fucopyranosyl ester (polygalasaponin L), in addition to five known compounds have been isolated from the roots of Polygala japonica.
New acylated triterpene saponins from Polygala tenuifolia willd.[Pubmed:16931424]
J Asian Nat Prod Res. 2006 Sep;8(6):499-503.
Two new acylated Presenegenin glycosides E-onjisaponin H (5) and Z-onjisaponin (6) together with seven known saponins were isolated from the roots of Polygala tenuifolia Willd. Compounds 5 and 6 were obtained as a pair of isomers due to trans and cis-p-methoxycinnamoyl. Their structures were elucidated mainly by 2D-NMR techniques including 1H-1HCOSY, TOCSY, HSQC, HMBC as 3-O-(beta-D-glucopyranosyl) Presenegenin 28-[O-beta-D-apiofuranosyl-(1 --> 3)-O-[beta-D-xylopyranosyl-(1 --> 4)]-O-alpha-L-rhamnopyranosyl-(1 --> 2)-O-[alpha-L-rhamnopyranosyl-(1 --> 3)]-4-O-[(E)-p-methoxycinnamoyl]-beta-D-fucopyranosyl] ester (5) and its (Z)-isomer (6).
[Study on the antihyperlipidemia effective constituent of Polygala fallax Hemsl].[Pubmed:16722311]
Zhong Yao Cai. 2006 Jan;29(1):16-9.
OBJECTIVE: To study the antihyperlipidemia effective constituent of Polygala fallax Hemsl. METHOD: Column chromatographic techniques were employed for isolation and purification of chemical constituents of the plant and the structures were elucidated by IR, NMR and MS spectroscopy. RESULT: Four triterpenoid saponins were isolated and determined as 3-O-beta-D-glucopyranosyl-( 1--> 2) -beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4 ) -alpha-L-rhamnopyranosyl-(1-->2 ) -beta-D-fucopyranosyl ester (I), 3-O-beta-D-glucopyranosyl-(1-->2) -beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4) -alpha-L-rhamnopyranosyl-(1-->2) -(3-O-acetyl)-beta-D-fucopyranosyl ester (II), 3-O-beta-D-glucopyranosyl-(1-->2) -beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4) -alpha-L-rhamnopyranosyl-(1-->2) -(4-O-acetyl) -beta-D-fucopyranosyl ester (II) and 3-O-beta-D-glucopyranosyl-(1--2) -beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4 ) -alpha-L-rhamnopyranosyl-(1-->2 ) - (3,4-diacetyl) -beta-D-fucopyranosyl ester (IV). CONCLUSIONS: The effective constituents of Polygala fallax Hemsl. reduced blood lipid especially plasma TG markedly. The authors have studied the chemical constituents of effective constituent systematically for the first time.
[Studies on chemical constituents in roots of Polygala tenuifolia].[Pubmed:15015373]
Zhongguo Zhong Yao Za Zhi. 2003 Sep;28(9):828-30.
OBJECTIVE: To study the chemical constituents from the roots of Polygala tenuifolia. METHOD: Column chromatographic techniques were employed for isolation and purification of chemical constituents of the plant and the structures were elucidated by spectroscopic analysis. RESULT: Five chemical constituents were isolated and elucidated as 4-C-beta-glucopyranosyl-1,3,6-trihydroxy-7-methoxyxanthone (1), 4-C-[beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranosyl]-1,3,6- trihydroxy-7-methoxyxanthone (2), Presenegenin (3), Presenegenin-3-O-beta-D-glycopyranoside (4) and daucosterol (5), respectively. CONCLUSION: Compounds 1,3,4 and 5 were isolated from this plant for the first time. Compound 1 is a new natural product.
Biologically active triterpene saponins from callus tissue of Polygala amarella.[Pubmed:10395523]
J Nat Prod. 1999 Jun;62(6):923-6.
A new bioactive saponin (1), together with a known saponin (polygalasaponin XXVIII) has been isolated from the callus tissue culture of Polygala amarella. Based on spectroscopic data, especially direct and long-range heteronuclear 2D NMR analysis and on chemical transformations, the structure of 1 was elucidated as 3-O-beta-D-glucopyranosyl Presenegenin-28-O-beta-D-galactopyranosyl-(1 --> 3)-beta-D-xylopyranosyl-(1 --> 4)-alpha-L-rhamnopyranosyl-(1 --> 2)-[beta-D-glucopyranosyl-(1 --> 3)]-beta-D-fucopyranoside. Both saponins showed significant immunological properties based on the enhancement of granulocyte phagocytosis in vitro.
Polygalasaponins XLII-XLVI from roots of Polygala glomerata.[Pubmed:9433820]
Phytochemistry. 1998 Feb;47(3):459-66.
Five new oleanane-type saponins, polygalasaponins XLII-XLVI, along with two known saponins were isolated from the roots of Polygala glomerata Lour. The structures of polygalasaponins XLII-XLVI were elucidated as 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl- (1-->2)-4-O-[(E)-3,4-dimethoxycinnamoyl]-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)- alpha-L-rhamnopyranosyl-(1-->2)-[alpha-L-arabinopyranosyl- (1-->3)]-[4-O-(E)-p-methoxycinnamoyl]-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)- alpha-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyranosyl- (1-->3)]-4-O-[(E)-3,4-dimethoxycinnamoyl]-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)- alpha-L-rhamnopyranosyl-(1-->2)-[6-O- acetyl-beta-D-glucopyranosyl-(1-->3)]-4-O- [(E)-3,4-dimethoxycinnamoyl]-beta-D-fucopyranosyl ester, 3-O-beta-D- glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)- beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl- (1-->2)-[6-O-acetyl-beta-D-glucopyranosyl-(1-->3)]-4-O-[(Z)-3, 4-dimethoxycinnamoyl]-beta-D-fucopyranosyl ester, respectively, on the basis of spectroscopic and chemical evidence.
Nine new triterpene saponins, polygalasaponins XXXIII--XLI from the roots of Polygala fallax Hemsl.[Pubmed:8945775]
Chem Pharm Bull (Tokyo). 1996 Nov;44(11):2092-9.
Nine new oleanane-type saponins, polygalasaponins XXXIII--XLI, along with seven known saponins were isolated from the roots of Polygala fallax HEMSL. Polygalasaponins XXXIII-XLI were elucidated as 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl- (1-->2)-(4-O-acetyl)-beta-D-fuco-pyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl- (1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-(4-O-acetyl)-beta-D- fucopyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)- alpha-L-rhamnopyranosyl-(1-->2)-(3,4-di-O-acetyl)-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)- [(5-O-acetyl)-beta-D-apiofuranosyl-(1-->3)]-alpha-L-rhamnopyranosy l- (1-->2)-(3,4-di-O-acetyl)-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->2)-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)- (3-O-acetyl)-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl- (1-->2)-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl- (1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-(4-O-acetyl)-beta-D- fucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->2)-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)- [alpha-L-rhamnopyranosyl-(1-->3)]-(4-O-acetyl)-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->2)-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)- [beta-D-apiofuranosyl-(1-->3)]-alpha-L-rhamnopyranosyl-(1-->2)- (3,4-di-O-acetyl)-beta-D-fucopyranosyl ester and 3-O-beta-D-glucopyranosyl-(1-->2)-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl-(1-->4)-beta-D-xylopyranosyl-(1-->4)- [(5-O-acetyl)-beta-D-apiofuranosyl-(-->3)]-alpha-L-rhamnopyranosyl - (1-->2)-(3,4-di-O-acetyl)-beta-D-fucopyranosyl ester, respectively, on the basis of spectroscopic and chemical evidence.
Five new triterpene saponins, polygalasaponins XXVIII-XXXII from the root of Polygala japonica Houtt.[Pubmed:8681413]
Chem Pharm Bull (Tokyo). 1996 Apr;44(4):810-5.
Five new oleanane-type saponins, polygalasaponins XXVIII-XXXII, along with one known saponin, polygalasaponin XXIV, and one known acylated sucrose, tenuifoliside C, were isolated from the root of Polygala japonica. The structures of these new compounds were elucidated as 3-O-beta-D-glucopyranosyl pesenegenin 28-O-beta-D-xylopyranosyl (1-->4)-alpha-L-rhamnopyranosyl (1-->2)-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl (1-->5)-beta-D-apiofuranosyl (1-->4)-beta-D-xylopyranosyl (1-->4)-alpha-L-rhamno-pyranosyl (1-->2)-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-beta-D-galactopyranosyl (1-->4)-beta-D-xylopyranosyl (1-->4)-alpha-L-rhamnopyranosyl (1-->2)-[4-O-p-methoxycinnamoyl]-[beta-D-glucopyranosyl (1-->3)]-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl Presenegenin 28-O-alpha-L-arabinopyranosyl (1-->3)-beta-D-xylopyranosyl (1-->4)-[beta-D-apiofuranosyl (1-->3)]-alpha-L-rhamnopyranosyl (1-->2)-[4-O-3,4,5-trimethoxycinnamoyl]-beta-D-fucopyranosyl ester, 3-O-beta-D-glucopyranosyl persenegenin 28-O-alpha-L-arabinopyranosyl (1-->3)-beta-D-xylopyranosyl (1-->4)-[beta-D-apiofuranosyl (1-->3)-alpha-L-rhamnopyranosyl (1-->2)-[4-O-p-methoxycinnamoyl]-[alpha-L-rhamnopyranosyl (1-->3)-beta-D-fucopyranosyl ester, respectively, on the basis of spectroscopic and chemical evidence.
Six new presenegenin glycosides, reiniosides A--F, from Polygala reinii root.[Pubmed:7774030]
Chem Pharm Bull (Tokyo). 1995 Mar;43(3):466-72.
Six new oleanane-type triterpene saponins, called reiniosides A-F, were isolated from the roots of Polygala reinii Fr. et Sav. and their structures were elucidated by spectroscopic and chemical means.


