Prednisolone AcetateCAS# 52-21-1 |
- BMY 14802 hydrochloride
Catalog No.:BCC5759
CAS No.:105565-55-7
- BD 1008 dihydrobromide
Catalog No.:BCC6674
CAS No.:138356-09-9
- BD 1047 dihydrobromide
Catalog No.:BCC6863
CAS No.:138356-21-5
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
- BD 1063 dihydrochloride
Catalog No.:BCC6832
CAS No.:206996-13-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 52-21-1 | SDF | Download SDF |
| PubChem ID | 5834 | Appearance | Powder |
| Formula | C23H30O6 | M.Wt | 402.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (124.23 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
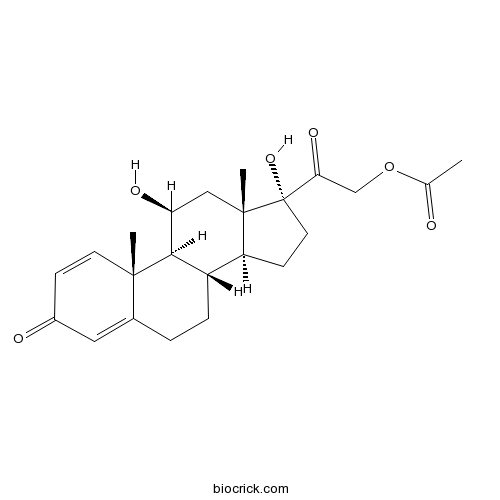
| Chemical Name | [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate | ||
| SMILES | CC(=O)OCC(=O)C1(CCC2C1(CC(C3C2CCC4=CC(=O)C=CC34C)O)C)O | ||
| Standard InChIKey | LRJOMUJRLNCICJ-JZYPGELDSA-N | ||
| Standard InChI | InChI=1S/C23H30O6/c1-13(24)29-12-19(27)23(28)9-7-17-16-5-4-14-10-15(25)6-8-21(14,2)20(16)18(26)11-22(17,23)3/h6,8,10,16-18,20,26,28H,4-5,7,9,11-12H2,1-3H3/t16-,17-,18-,20+,21-,22-,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Prednisolone 21-acetate is an adrenal cortico hormones, with anti-inflammatory, anti-allergic and immune suppressive effects. |

Prednisolone Acetate Dilution Calculator

Prednisolone Acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4846 mL | 12.423 mL | 24.846 mL | 49.6919 mL | 62.1149 mL |
| 5 mM | 0.4969 mL | 2.4846 mL | 4.9692 mL | 9.9384 mL | 12.423 mL |
| 10 mM | 0.2485 mL | 1.2423 mL | 2.4846 mL | 4.9692 mL | 6.2115 mL |
| 50 mM | 0.0497 mL | 0.2485 mL | 0.4969 mL | 0.9938 mL | 1.2423 mL |
| 100 mM | 0.0248 mL | 0.1242 mL | 0.2485 mL | 0.4969 mL | 0.6211 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Prednisolone Acetate
- Spironolactone
Catalog No.:BCC4366
CAS No.:52-01-7
- Dehydroespeletone
Catalog No.:BCN5652
CAS No.:51995-99-4
- Schaftoside
Catalog No.:BCN2343
CAS No.:51938-32-0
- 5-Aminoindole
Catalog No.:BCC8735
CAS No.:5192-03-0
- TNP
Catalog No.:BCC7822
CAS No.:519178-28-0
- CJ 033466
Catalog No.:BCC7562
CAS No.:519148-48-2
- Tasisulam
Catalog No.:BCC4407
CAS No.:519055-62-0
- Dehydrohautriwaic acid
Catalog No.:BCN7586
CAS No.:51905-84-1
- 4-(N-Methyl)-aminoantipyrine
Catalog No.:BCC8652
CAS No.:519-98-2
- Sulochrin
Catalog No.:BCN6959
CAS No.:519-57-3
- Maclurin
Catalog No.:BCN5651
CAS No.:519-34-6
- Ellipticine
Catalog No.:BCC7665
CAS No.:519-23-3
- Thio-TEPA
Catalog No.:BCC5354
CAS No.:52-24-4
- Morphine hydrochloride
Catalog No.:BCC6368
CAS No.:52-26-6
- H-D-Pen-OH
Catalog No.:BCC3307
CAS No.:52-67-5
- Lynestrenol
Catalog No.:BCC9014
CAS No.:52-76-6
- Haloperidol
Catalog No.:BCC4909
CAS No.:52-86-8
- H-Cys-OH
Catalog No.:BCC2902
CAS No.:52-90-4
- 6-Methoxyluteolin
Catalog No.:BCN3613
CAS No.:520-11-6
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- Kaempferol
Catalog No.:BCN5653
CAS No.:520-18-3
- Hesperidin
Catalog No.:BCN5654
CAS No.:520-26-3
- Diosimin
Catalog No.:BCN4993
CAS No.:520-27-4
- Tectochrysin
Catalog No.:BCN5655
CAS No.:520-28-5
A retrospective analysis of intraocular pressure changes after cataract surgery with the use of prednisolone acetate 1% versus difluprednate 0.05%.[Pubmed:27920493]
Clin Ophthalmol. 2016 Nov 23;10:2329-2336.
PURPOSE: To compare the effect of topical Prednisolone Acetate 1% (PA) used after routine cataract surgery to the effect of difluprednate 0.05% (DFBA) used for the same indication on intraocular pressure (IOP). METHODS: An electronic query was created to gather information from all cataract surgeries between January 2010 and January 2015 within the electronic health record database at Barnet Dulaney Perkins, a multicenter, multiphysician private practice in Phoenix, Arizona. Information collected included age, sex, diabetes status, glaucoma history, medication regimen (use of PA or DFBA), and IOP before surgery, 5-10 days postoperatively (TP1) and 3-6 weeks postoperatively (TP2). Postoperative IOP measurements were compared to baseline IOP measurement in each patient. RESULTS: Regardless of steroid used, all patients in this study experienced an increase in IOP within TP1 and returned to baseline IOP (+/-2.0 mmHg) by TP2. Patients who received DFBA showed a statistically significant increase in IOP at TP1 compared to those on PA (P<0.001) with the mean IOP an average 0.60 mmHg higher (95% CI =0.3, 0.9). The odds ratio of a clinically significantly increased IOP at TP1 (defined as overall IOP >/=21 mmHg and an increase of >/=10 mmHg) in DFBA-treated patients was 1.84 (95% CI =1.4, 2.6). In patients treated with PA, 3% reached a significantly increased IOP, compared to 4.4% of patients in the DFBA group (P<0.05). Risk factors for increased IOP were identified, and include advanced age (>75) (P<0.005) and a history of glaucoma (P<0.001). CONCLUSION: In postoperative cataract patients, use of DFBA increased the risk of a clinically significant IOP increase.
Comparison of prednisolone acetate 1.0% and difluprednate ophthalmic emulsion 0.05% after cataract surgery: Incidence of postoperative steroid-induced ocular hypertension.[Pubmed:28366370]
J Cataract Refract Surg. 2017 Feb;43(2):223-227.
PURPOSE: To compare intraocular pressure (IOP) outcomes between 2 common, commercially available corticosteroid drops: difluprednate ophthalmic emulsion 0.05% and Prednisolone Acetate 1.0%. SETTING: TLC Eyecare and Laser Centers, Jackson, Michigan, USA. DESIGN: Retrospective chart review. METHODS: The outcomes of consecutive patients who had uneventful cataract surgery from April 2013 to September 2013 and used Prednisolone Acetate postoperatively were compared with the outcomes of consecutive patients who had uneventful cataract surgery from June 2014 to October 2014 and used difluprednate postoperatively. RESULTS: The study included 224 eyes treated with Prednisolone Acetate 4 times daily for 30 days and 225 eyes treated with difluprednate 2 times daily for 30 days. There was no significant difference between the 2 groups in age, sex, or race. In addition, the mean IOP did not differ significantly between the Prednisolone Acetate group and the difluprednate group at the preoperative measurement or 1 month after surgery, nor was there a difference in the 1-month change in IOP between groups. No association was found between the incidence of a 6 mm Hg or higher increase in IOP 1 month after surgery and steroid treatment. One month postoperatively, 4 eyes in the Prednisolone Acetate group and 5 eyes in the difluprednate group had an IOP higher than 21 mm Hg. CONCLUSIONS: There was no significant difference in the mean IOP or percentages showing IOP elevation between eyes treated with difluprednate and eyes treated with Prednisolone Acetate after cataract surgery. This was likely the result of low-frequency dosing and short duration of steroid use.
To Study the Efficacy of Difluprednate Ophthalmic Emulsion and Prednisolone Acetate Ophthalmic Suspension on Post-operative Inflammation in Cataract Surgery.[Pubmed:28208898]
J Clin Diagn Res. 2016 Dec;10(12):NC05-NC08.
INTRODUCTION: Senile cataract is the most common cause of visual impairment. Removal of cataract and implantation of intraocular lens implantation (IOL) is the main surgical approach for cataract. The major block in quick visual rehabilitation of the patient is post-operative inflammation. To limit post-operative inflammation corticosteroids drugs are used in routine prophylactically. Topical Prednisolone Acetate 1% and betamethasone 0.1% remain gold standard to control post-operative inflammation but newer drugs like difluprednate, loteprednol are also effective in controlling inflammation. AIM: To study the efficacy of difluprednate ophthalmic emulsion and Prednisolone Acetate ophthalmic suspension on Post-operative inflammation in cataract surgery (clear corneal phacoemulsification with foldable IOL). MATERIALS AND METHODS: This study was carried out on 100 patients having visually significant cataract requiring surgery, clear corneal phacoemulsification with foldable intraocular lens implantation was done in all patients. Patients were randomly divided into two groups. In group A topical 1% Prednisolone Acetate ophthalmic suspension was administered six times a day Post-operatively. In group B 0.05% difluprednate ophthalmic emulsion was administered six times a day post-operatively. Efficacy of drug was evaluated in terms of decrease in ocular pain, anterior chamber reaction in the form of aqueous cells and flare and final visual acuity at 4 weeks. RESULTS: In this study, 92% of patients in group A and 90% of patients in group B had BCVA 6/6. None of the patients in group A had ocular pain. In group B, 96% patients had no ocular pain. Remaining 4% had mild discomfort but required no medication. 98% of patients in group A and 100% of patients in group B presented with clearance of aqueous cells at the end of study. Only 2% of patients in group A had showed cell score (+/-). CONCLUSION: Though Prednisolone Acetate has been the gold standard anti inflammatory agent, 0.05% Difluprednate ophthalmic emulsion is equally effective in treatment of post-operative inflammation. Difluprednate have added an advantage of uniform drug dosage and absence of harmful preservative.
Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol((R)) gel under Dermaroller((R)) on rats with methyl prednisolone acetate-induced hypertension.[Pubmed:28237913]
Biomed Pharmacother. 2017 May;89:177-184.
The objective of present study was to prepare eprosartan mesylate (EM)-loaded transfersomes Carbopol((R)) gel and characterized for various parameters, including in vitro skin permeation, in vivo antihypertensive study, skin irritation, and histological study. Furthermore, effect of transfersomes gel on angiotensin II type-1 receptor (AT1R) mRNA and protein expressions on smooth vascular muscles of aorta was determined by real-time polymerase chain reaction (RT-PCR) and western blot analysis. The physical evaluation parameters were detected to be in correspondence with reference marketed gel formulation. The transdermal flux, permeability coefficient, and Tlag of EM from transfersomes gel were found to be 26.76 +/- 1.66mug/cm(2)/h, 8.93 +/- 0.55 x10(-3) cm/h, and 2.17 +/- 0.29h, respectively, across rat skin pretreated with microneedle (Dermaroller((R))). Pharmacodynamic study showed prolonged and better management of hypertension after the application of transfersomes gel in experimentally induced hypertensive Wistar rats as compared with oral control formulation. The in vivo angiotensin II type-1 blocking efficacy of prepared transfersomes gel and control formulation was also supported with RT-PCR and western blot analysis of AT1R mRNA and protein expressions on smooth vascular muscles of aorta. Skin irritation and skin histological assessment showed that the prepared transfersomes Carbopol((R)) gel was safe to be used for transdermal route. It is concluded that the incorporation of transfersomes into gel formulation offered enhanced skin contact, ease of application, and found to be a suitable drug reservoir for the transdermal delivery of EM for the management of hypertension in Wistar rats.


