Polygalaxanthone IIICAS# 162857-78-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 162857-78-5 | SDF | Download SDF |
| PubChem ID | 11169063 | Appearance | Powder |
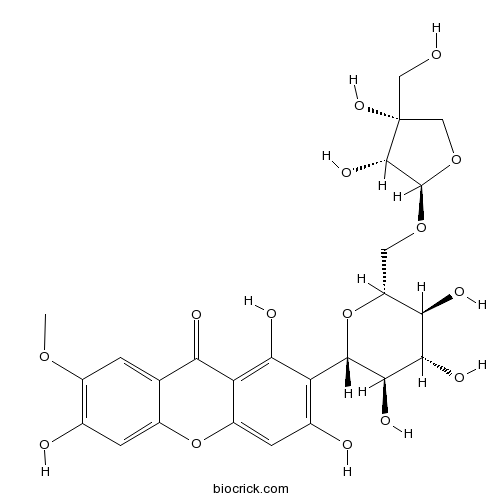
| Formula | C25H28O15 | M.Wt | 568.39 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (439.77 mM; Need ultrasonic) | ||
| Chemical Name | 2-[(2S,3R,4R,5S,6R)-6-[[(2R,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxymethyl]-3,4,5-trihydroxyoxan-2-yl]-1,3,6-trihydroxy-7-methoxyxanthen-9-one | ||
| SMILES | COC1=C(C=C2C(=C1)C(=O)C3=C(C(=C(C=C3O2)O)C4C(C(C(C(O4)COC5C(C(CO5)(CO)O)O)O)O)O)O)O | ||
| Standard InChIKey | LLJUCXMVRPAONX-AYATVRNWSA-N | ||
| Standard InChI | InChI=1S/C25H28O15/c1-36-12-2-8-11(3-9(12)27)39-13-4-10(28)15(19(31)16(13)17(8)29)22-21(33)20(32)18(30)14(40-22)5-37-24-23(34)25(35,6-26)7-38-24/h2-4,14,18,20-24,26-28,30-35H,5-7H2,1H3/t14-,18-,20+,21-,22+,23+,24-,25-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Polygalaxanthone III can significantly inhibit chlorzoxazone 6-hydroxylation catalyzed by CYP2E1. |
| Targets | P450 (e.g. CYP17) |
| In vitro | [Effect of oligosaccharide esters and polygalaxanthone Ill from Polygala tenuifolia willd towards cytochrome P450].[Pubmed: 25850285]Zhongguo Zhong Yao Za Zhi. 2014 Nov;39(22):4459-63.
|
| Structure Identification | J Mass Spectrom. 2013 Aug;48(8):904-13.A UFLC-MS/MS method with a switching ionization mode for simultaneous quantitation of polygalaxanthone III, four ginsenosides and tumulosic acid in rat plasma: application to a comparative pharmacokinetic study in normal and Alzheimer's disease rats.[Pubmed: 23893636]
|

Polygalaxanthone III Dilution Calculator

Polygalaxanthone III Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7594 mL | 8.7968 mL | 17.5936 mL | 35.1871 mL | 43.9839 mL |
| 5 mM | 0.3519 mL | 1.7594 mL | 3.5187 mL | 7.0374 mL | 8.7968 mL |
| 10 mM | 0.1759 mL | 0.8797 mL | 1.7594 mL | 3.5187 mL | 4.3984 mL |
| 50 mM | 0.0352 mL | 0.1759 mL | 0.3519 mL | 0.7037 mL | 0.8797 mL |
| 100 mM | 0.0176 mL | 0.088 mL | 0.1759 mL | 0.3519 mL | 0.4398 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Polygalasaponin V
Catalog No.:BCN2790
CAS No.:162857-65-0
- 2,3,9,10-Tetrahydroxyberberine
Catalog No.:BCN3550
CAS No.:162854-37-7
- IEM 1754 dihydrobroMide
Catalog No.:BCC5049
CAS No.:162831-31-4
- 7-Epi-10-oxo-docetaxel
Catalog No.:BCC5410
CAS No.:162784-72-7
- LJI308
Catalog No.:BCC6538
CAS No.:1627709-94-7
- PFI-2
Catalog No.:BCC5561
CAS No.:1627676-59-8
- Yunnancoronarin A
Catalog No.:BCN1723
CAS No.:162762-93-8
- WAY-100635
Catalog No.:BCC2053
CAS No.:162760-96-5
- Anemarrhena B
Catalog No.:BCN7592
CAS No.:1627521-95-2
- LDN-214117
Catalog No.:BCC5528
CAS No.:1627503-67-6
- AZD8186
Catalog No.:BCC6470
CAS No.:1627494-13-6
- 1-Hydroxy-2-prenylnaphthalene
Catalog No.:BCN1722
CAS No.:16274-34-3
- Antibiotic AB 4015B
Catalog No.:BCN1826
CAS No.:162857-79-6
- (S)-3,5-DHPG
Catalog No.:BCC6802
CAS No.:162870-29-3
- Kaempferol-7-O-D-glucopyranoside
Catalog No.:BCN2296
CAS No.:16290-07-6
- 3,4-Dihydroxybenzylamine Hydrobromide
Catalog No.:BCC8280
CAS No.:16290-26-9
- Bis(4-bromophenyl)amine
Catalog No.:BCC8883
CAS No.:16292-17-4
- K-Ras G12C-IN-1
Catalog No.:BCC5538
CAS No.:1629265-17-3
- K-Ras G12C-IN-2
Catalog No.:BCC5539
CAS No.:1629267-75-9
- K-Ras G12C-IN-3
Catalog No.:BCC5540
CAS No.:1629268-19-4
- 3-O-(2-Aminoethyl)-25-hydroxyvitamin D3
Catalog No.:BCC1309
CAS No.:163018-26-6
- 2-Cl-IB-MECA
Catalog No.:BCC6938
CAS No.:163042-96-4
- Cimicifugoside H1
Catalog No.:BCN7950
CAS No.:163046-73-9
- Lup-20(29)-ene-3bate,23-diol
Catalog No.:BCN4080
CAS No.:163060-07-9
A UFLC-MS/MS method with a switching ionization mode for simultaneous quantitation of polygalaxanthone III, four ginsenosides and tumulosic acid in rat plasma: application to a comparative pharmacokinetic study in normal and Alzheimer's disease rats.[Pubmed:23893636]
J Mass Spectrom. 2013 Aug;48(8):904-13.
A fast, sensitive and reliable ultra fast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS) method has been developed and validated for simultaneous quantitation of Polygalaxanthone III (POL), ginsenoside Rb1 (GRb1), ginsenoside Rd (GRd), ginsenoside Re (GRe), ginsenoside Rg1 (GRg1) and tumulosic acid (TUM) in rat plasma after oral administration of Kai-Xin-San, which plays an important role for the treatment of Alzheimer's disease (AD). The plasma samples were extracted by liquid-liquid extraction using ethyl acetate-isopropanol (1:1, v/v) with salidrdoside as internal standard (IS). Good chromatographic separation was achieved using gradient elution with the mobile phase consisting of methanol and 0.01% acetic acid in water. The tandem mass spectrometric detection was performed in multiple reaction monitoring mode on 4000Q UFLC-MS/MS system with turbo ion spray source in a negative and positive switching ionization mode. The lower limits of quantification were 0.2-1.5 ng/ml for all the analytes. Both intra-day and inter-day precision and accuracy of analytes were well within acceptance criteria (+/-15%). The mean absolute extraction recoveries of analytes and IS from rat plasma were all more than 60.0%. The validated method has been successfully applied to comparing pharmacokinetic profiles of analytes in normal and AD rat plasma. The results indicated that no significant differences in pharmacokinetic parameters of GRe, GRg1 and TUM were observed between the two groups, while the absorption of POL and GRd in AD group were significantly higher than those in normal group; moreover, the GRb1 absorbed more rapidly in model group. The different characters of pharmacokinetics might be caused by pharmacological effects of the analytes.
[Effect of oligosaccharide esters and polygalaxanthone Ill from Polygala tenuifolia willd towards cytochrome P450].[Pubmed:25850285]
Zhongguo Zhong Yao Za Zhi. 2014 Nov;39(22):4459-63.
Five compounds (tenuifoliside C, tenuifoliside D, telephiose A, telephiose C and Polygalaxanthone III) from polygala tenuifolia wild were incubated together with CYP probe substrate in human liver microsomes to investigate the inhibitory effect towards CYP450 enzyme. Phenacetin (CYP1A2), coumarin (CYP2A6), paclitaxel (CYP2C8), diclofenac (CYP2C9), S-mepheriytoin (CYP2C19), dextromethorphan (CYP2D6), chlorzoxazone (CYP2E1), midazolam (CYP3A) were selected as the isoforfn specific substrate. And the formation of paracetamol, 7-hydroxycoumarin, 6alpha-hydroxy paclitaxel, 4'-hydroxydiclofenac, dextrorphan, 6-hydroxychlorzoxazone, 1'-hydroxymidazolam, 4'-hydroxymephenytoin were detected respectively to measure the effect towards CYP450 by high-pressure liquid chromatography (HPLC). The result shows that five compounds from polygala tenuifolia willd significantly inhibit chlorzoxazone 6-hydroxylation catalyzed by CYP2E1, while showed no effect towards CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A. And IC50 value was 38.73, 54.14, 61.77, 62.22, 50.56 micromol x L(-1), respectively.
Simultaneous quantitation of polygalaxanthone III and four ginsenosides by ultra-fast liquid chromatography with tandem mass spectrometry in rat and beagle dog plasma after oral administration of Kai-Xin-San: application to a comparative pharmacokinetic study.[Pubmed:24610822]
J Sep Sci. 2014 May;37(9-10):1103-10.
A fast, selective, and quantitative ultra-fast liquid chromatography with tandem mass spectrometry method has been developed and validated for the simultaneous quantitation of Polygalaxanthone III, ginsenoside Rb1, ginsenoside Rd, ginsenoside Re, and ginsenoside Rg1 in the plasma of rat and beagle dog after oral administration of Kai-Xin-San. After addition of the internal standard, salidroside, the plasma samples were extracted by liquid-liquid extraction and separated on a Venusil MP C18 column with methanol/0.01% acetic acid water as mobile phase. The tandem mass spectrometric detection was performed in the multiple reaction monitoring with turbo ion spray source in a switching ionization mode. The method was examined, and found to be precise and accurate with the linearity range of the compounds. The intra- and interday precision and accuracy of the analytes were well within acceptance criteria (+/-15%). The mean extraction recoveries of analytes and internal standard were all >75.0%. The validated method has been successfully applied to comparing pharmacokinetic profiles of analytes in rat and beagle dog plasma. The results indicated that no significant differences were observed in pharmacokinetic parameters of ginsenoside Rg1, while the others had significant differences, which may due to the different mechanisms of absorption and metabolism.


